Hypothetical Model for REDs Endocrine Disruptions
Introduction
Persistent or severe low energy availability (what is termed ‘problematic’ LEA) places an athlete at risk for the development of Relative Energy Deficiency in Sports (REDs) which compromises both their health and performance capacity (1). Studies indicate LEA/REDs are associated with hormonal disturbances in male and female athletes (1). These disturbances are especially noted in the reproductive sex steroid hormones (i.e., reduced levels of testosterone [T], oestradiol-β-17 [E2], and progesterone [P4]) (2). Additionally, evidence supports the development of low triiodothyronine (T3) profiles in LEA/REDs athletes (2,3). Recent work suggests the presence of low carbohydrate (LCHO) intake coupled with LEA exacerbates the likelihood of REDs symptoms developing (1,4). This blog presents a hypothetical model of how LCHO dietary intake and LEA interact to create an amplification of the endocrine dysfunctions associated with REDs
What’s Carbohydrates Endocrine Connection?
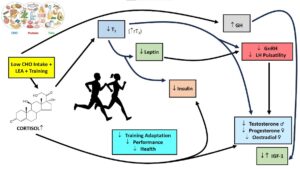
Over 40 years ago Galbo et al. demonstrated that as little as four days of a low carbohydrate diet intensified the exercise cortisol response (5). Cortisol, whilst an essential hormone in the human internal milieu, has a cascading series of direct-indirect agonist-antagonist detrimental actions on other hormones (6,7). Figure 1 shows a hypothetical model of some of the hormone interactions and the effects of cortisol exposure.
Chronic Repetitive exposure
Researchers often assume persistent, chronic exposure to a hormone such as cortisol, is necessary to invoke the changes demonstrated in Figure 1. In actual fact, this is only one scenario. Elite and semi-elite athletes train almost daily and often have multiple sessions per day. Most such training sessions will invoke repetitive exposures to elevated cortisol levels (albeit transient, proportional to training intensity, and can approach supraphysiological levels) (8). These frequent exposures to daily cortisol elevations can potentially have an additive effect similar to that of chronic elevations (6,9,10).
Figure 1. Hypothetical model of how low energy availability (LEA) plus low carbohydrate intake creates an amplification effect on creating a hormonal profile associated with problematic LEA and Relative Energy Deficiency in Sports (REDs) (16).
Criticality of Triiodothyronine (T3)
In the model depicted in Figure 1, T3 plays a critical role due to its permissive actions (i.e., helping in the function of other hormones) (11). The reduction of T3 driven by LEA (N.B., caloric restriction/reduction suppresses T3) is intensified by cortisol activation of the 5’deiodinase enzyme activity resulting in the prohormone thyroxine and T3 conversion to inactive metabolites, principally 3,3′,5′-triiodothyronine (reverseT3 [rT3]) (11). The lack of T3, which functionally is more bioactive (i.e., brings about changes in tissues), leads to the amplification of T, E2, and P4 reductions already occurring due to the regulatory axis controlling their production being disrupted (see Figure 1 and Table 1 [HPG Axis]). These key reproductive hormones are further compromised via cortisol’s direct inhibition of their production (steroidogenesis inhibition) as well as its similar inhibitory actions on leptin and insulin (7, 11-14). Additionally, while cortisol and growth hormone (GH) linkage is complicated, glucocorticoids such as cortisol can inhibit somatostatin (GH inhibiting hormone) gene expression and cause circulating GH to increase as well as subsequently leading to altered insulin-like growth factor (IGF) levels (15).
Conclusions
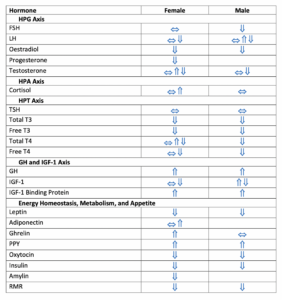
In their excellent review article, Elliott-Sale and associates summarized the endocrine profiles of female and male athletes with LEA/REDs (2). Table 1 presents a summary of their key findings. While the model presented in Figure 1 does not capture all facets of the hormonal changes reported by these authors, it does represent the major ones generally reported in REDs literature (1).
Table 1. Summary of the hormonal changes associated with low energy availability and Relative Energy Deficiency in Sports (REDs) as reported by Elliott-Sale et al. (2). Legend: ⇓ = decrease, ⇑ = increase, ⇔ = no change. Abbreviations: HPG = hypothalamic-pituitary-gonadal, FSH = follicle-stimulating hormones, LH = luteinizing hormone, HPA = hypothalamic-pituitary-adrenal, HPT = hypothalamic-pituitary-thyroid, TSH = thyroid stimulating hormone, T3 = triiodothyronine, T4 = thyroxine, GH = growth hormone, IGF = insulin-like growth factor, PPY = peptide YY, RMR = resting metabolic rate (non-hormonal measure) (2).
There are two key take-home messages here:
- the combined impact of problematic LEA and low CHO intake potentially creates an amplification of the hormone profile associated with compromise adaptation, performance, and health in athletes; and,
- investigators studying LEA/REDs need to be more multidimensional and encompassing in their research relative to hormonal interactions, to allow the model proposed here, to be confirmed or denied.
Practically speaking, it is critical for athletes to recognize the importance of carbohydrates in their diets. Regrettably, the fear of carbohydrates and the practice of “carb shaming” due to concerns about body weight and body composition exist in sports and are tangible problems for both females and males. Proper dietary practices to allow adequate energy intake (“fueling”) involving all nutrients (especially carbohydrates) is essential to prevent LEA/REDs and allow athletes to have proper adaptations to their training and ultimately improve their performance.
Author Affiliation
Anthony C. Hackney
University of North Carolina
Twitter @AC_Hackney
References
- Mountjoy ML, Ackerman KE, Bailey DM, Burke LM, Constantini N, Hackney AC, Heikura IA, Melin AK, Pensgaard AM, Stellingwerff T, Sundgot-Borgen J, Torstveit MK, Uhrenholdt Jacobsen A, Verhagen E, Budgett R, Engebretsen L, Erdener U. The 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sports (REDs). British J Sports Medicine. 2023 (in press).
- Elliott-Sale KJ, Tenforde AS, Parziale AL, Holtzman B, Ackerman KE. Endocrine Effects of Relative Energy Deficiency in Sport. Int J Sport Nutr Exerc Metab. 2018 Jul 1;28(4):335-349.
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015;25(5):610-22.
- Kojima C, Ishibashi A, Tanabe Y, et al. Muscle Glycogen Content during Endurance Training under Low Energy Availability. Med Sci Sports Exerc 2020;52(1):187-95.
- Galbo H, Holst JJ, Christensen NJ. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand. 1979 Sep;107(1):19-32.
- Viru A, Viru M. Cortisol–essential adaptation hormone in exercise. Int J Sports Med. 2004 Aug;25(6):461-4.
- St-Pierre DH, Richard D. The effect of exercise on the hypothalamic-pituitary-adrenal axis. In: A. C. Hackney, N. W. Constantini (eds.), Endocrinology of Physical Activity and Sport, Contemporary Endocrinology. https://doi.org/10.1007/978-3-030-33376-8_3. Springer, 2020.
- Daly W, Hackney AC. Is exercise cortisol response of endurance athletes similar to levels of Cushing’s syndrome? Biol Sport. 2005;22(3):209-214.
- Veldhuis JD, Yoshida K. Impact of chronic training on pituitary hormone secretion in humans. In: A. C. Hackney, N. W. Constantini (eds.), Endocrinology of Physical Activity and Sport, Contemporary Endocrinology. https://doi.org/10.1007/978-3-030-33376-8_3. Springer, 2020.
- Viru AM, Hackney AC, Välja E, Karelson K, Janson T, Viru M. Influence of prolonged continuous exercise on hormone responses to subsequent exercise in humans. Eur J Appl Physiol. 2001 Oct;85(6):578-85.
- Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers; 2001. Chapter 3, The thyroid gland. Available from: https: //www.ncbi.nlm.nih.gov/books/NBK28/
- Cumming DC, Wheeler GD, McColl EM. The effects of exercise on reproductive function in men. Sports Med. 1989 Jan;7(1):1-17.
- Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983 Sep;57(3):671-3.
- Adam TC, Hasson RE, Ventura EE, Toledo-Corral C, Le KA, Mahurkar S, Lane CJ, Weigensberg MJ, Goran MI. Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. J Clin Endocrinol Metab. 2010 Oct;95(10):4729-35.
- Liu JL, Patel YC. Glucocorticoids inhibit somatostatin gene expression through accelerated degradation of somatostatin messenger ribonucleic acid in human thyroid medullary carcinoma (TT) cells. Endocrinology. 1995 Jun;136(6):2389-96.
- Hackney AC. Energy availability: are there sex differences in hormonal responses? Presentation – Society of Endocrinology meeting, Nottingham Trent University, United Kingdom, July 2022.