Is the gut microbiome important when considering joint health in the athlete?
Keywords: Microbiome, gut-joint axis, athlete
Introduction
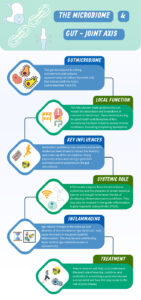
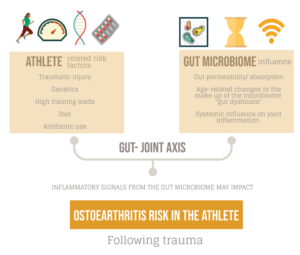
For the athlete or exercising patient, injury, training load, genetics, and training type have traditionally been thought of as the main factors that influence the progression of joint disease. Emerging evidence in the field of microbiome research has shown that a new risk factor may exist, and there are plausible mechanistic links in the gut-joint axis that could influence the initiation and progression of diseases such as ankylosing spondylitis (AS) and osteoarthritis (OA) (1,2). This blog explores how the gut microbiome may influence joint disease as well as age related disease progression (inflammaging) and how this is applied to the elite athlete.
Gut microbiome
The average human gut microbiome is a living diverse environment of microbes (bacteria, fungi, viruses etc) and by recent critically revised estimates contains approximately as many bacterial cells (~39 trillion) as human cells (~30 trillion), not taking into account the other microbial elements (3). This micro-environment is metabolically active and may play a critical role in the permeability and absorption of antigen and nutrients across the large interface of the gastrointestinal tract (GI) (250-400m2) (4). This unexplored ‘organ system’ forms a key relationship with the host and is impacted by factors such as exercise, diet, and antibiotic use. In the elite athlete group, high training loads, protein intake and dietary factors can influence the make-up and diversity of this microbiome and impact its key functions (5).
What is the gut joint axis? How can the gut joint axis impact the host systemically?
Recent research suggests that the gut microbiome may have a significant systemic impact on the host (gut-joint axis). Several studies have shown the microbiome may produce inflammatory and cytokine signals that travel into the host organism, and directly control joint inflammation (6,7). Early work in animal models shows that when a traumatic injury has occurred, the presence of certain bacterial species in the microbiome can modulate the development of early onset post traumatic OA (8). While still in the early stages, this work suggests that the gut microbiome could be key to systemic inflammation and the trajectory of disease progression in subtypes of (post traumatic) OA that may be relevant in the context of sports medicine.
Exercise and the gut microbiome: What do we know so far?
Observational studies have shown that exercise/physical activity & diet may play a key role in maintaining the microbiome. Several studies in athletic and normal populations have reported that those who are physically active may have a more diverse and “rich” gut microbiota compared to sedentary individuals (9). Due to limitations in these cross-sectional cohort studies, it is difficult to determine how much influence each individual factor (diet/exercise) has – or if the addition of probiotics could help to optimise the microbiome.
Inflammaging and gut dysbiosis: How could they impact joint health?
Age is considered a major risk factor for the development of OA and recent studies have suggested age-related changes occur in the microbiome over the course of an individual’s lifetime (“gut dysbiosis”) (10). The observed “gut dysbiosis”, may be part of a spectrum of changes to the make-up of the microbiome, where there is an imbalance in terms of the biodiversity and metabolic potential.
These changes have been linked to increased intestinal permeability, increased serum circulating inflammatory cytokines and matrix metalloproteases, that may contribute to systemic inflammation (9). This theory linking age-related microbiome changes to the progression of OA is known as “inflammaging” and could help us in the future to target new ways to reduce low grade systemic inflammation and prevent OA.
| Ankylosing Spondylitis and the gut joint axis what do we know so far? |
|
Currently there is promising research, looking at “gut dysbiosis” and the factors that slow down or prevent it from occurring in older age. Future research will help us to understand how physical activity, probiotics and appropriate nutrition may be used in regulating gut dysbiosis.
For the elite athlete at risk of traumatic joint injuries over their career, knowing if and how to reduce systemic inflammation or optimise signalling from the gut joint axis – may reduce their long-term risk of joint disease (post traumatic OA).
Is osteoarthritis more common in athletes?
Although some people have hypothesised that athletes may be at higher risk of OA, due to injury risk and training demands, there have been very few high-quality studies to determine if this is true. One systematic review suggested that there was an increased relationship between OA and elite sports, but that this was based on low quality evidence (15). It remains to be seen if microbiome focused interventions, either via diet or exercise, have the potential to be protective in populations at higher risk of OA or AS.
Conclusion
- The gut-joint axis is an exciting area of research in musculoskeletal medicine and has the potential to offer ways to reduce the long-term risk of joint disease with simple lifestyle interventions.
- At this stage studies are largely limited to animal models, but early evidence demonstrates that physical activity and dietary modification may have a significant impact on age-related joint conditions (inflammaging).
- Further research into gut dysbiosis, and the potential role of inflammatory signals from the gut microbiome would be invaluable.
- In the future, microbiome interventions may be used to modulate the risk of developing OA in high risk groups (post trauma) or patients at risk of AS.
Authors names & affiliations
Raj Amarnani (@DrRajAmar)
Sports & Exercise Medicine Registrar,
Imperial College Healthcare NHS Trust, London, United Kingdom
Irfan Ahmed (@Exerciseirfan)
Sport & Exercise Medicine Consultant,
Connect Health, United Kingdom
Madhura Castelino
Rheumatology Consultant,
University College London Hospitals NHS Foundation Trust, London, United Kingdom
The authors declare no competing interests
References
- Manasson J, Blank RB, Scher JU. The microbiome in rheumatology: Where are we and where should we go? Ann Rheum Dis [Internet]. 2020 Jun;79(6):727–33. Available from: https://ard.bmj.com/lookup/doi/10.1136/annrheumdis-2019-216631
- Hao X, Shang X, Liu J, Chi R, Zhang J, Xu T. The gut microbiota in osteoarthritis: where do we stand and what can we do? Arthritis Res Ther [Internet]. 2021 Dec 27;23(1):42. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-021-02427-9
- Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biol [Internet]. 2016 Aug 19;14(8):e1002533. Available from: https://dx.plos.org/10.1371/journal.pbio.1002533
- BENGMARK S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut [Internet]. 1998 Jan 1;42(1):2–7. Available from: https://gut.bmj.com/lookup/doi/10.1136/gut.42.1.2
- O’Donovan CM, Madigan SM, Garcia-Perez I, Rankin A, O’ Sullivan O, Cotter PD. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J Sci Med Sport [Internet]. 2020 Jan;23(1):63–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1440244019307339
- Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, et al. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res Rev [Internet]. 2019;55:100946. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31437484
- Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr Cartil [Internet]. 2016;24(10):1769–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27216281
- Ulici V, Kelley KL, Azcarate-Peril MA, Cleveland RJ, Sartor RB, Schwartz TA, et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr Cartil [Internet]. 2018;26(8):1098–109. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29857156
- Mohr AE, Jäger R, Carpenter KC, Kerksick CM, Purpura M, Townsend JR, et al. The athletic gut microbiota. J Int Soc Sports Nutr [Internet]. 2020 Dec 12;17(1):24. Available from: https://jissn.biomedcentral.com/articles/10.1186/s12970-020-00353-w
- Grosicki GJ, Fielding RA, Lustgarten MS. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif Tissue Int [Internet]. 2018 Apr 20;102(4):433–42. Available from: http://link.springer.com/10.1007/s00223-017-0345-5
- Asquith M, Elewaut D, Lin P, Rosenbaum JT. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract Res Clin Rheumatol [Internet]. 2014 Oct;28(5):687–702. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25488778
- Costello M-E, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, et al. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol (Hoboken, NJ) [Internet]. 2015 Mar;67(3):686–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25417597
- Voruganti A, Bowness P. New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology [Internet]. 2020 Oct 17;161(2):94–102. Available from: https://onlinelibrary.wiley.com/doi/10.1111/imm.13242
- Mielants H, De Vos M, Goemaere S, Schelstraete K, Cuvelier C, Goethals K, et al. Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J Rheumatol [Internet]. 1991 Mar;18(3):394–400. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1906939
- Tran G, Smith TO, Grice A, Kingsbury SR, McCrory P, Conaghan PG. Does sports participation (including level of performance and previous injury) increase risk of osteoarthritis? A systematic review and meta-analysis. Br J Sports Med [Internet]. 2016 Dec;50(23):1459–66. Available from: https://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2016-096142