How should we be investigating and monitoring bone health in the young athlete?
Bone health is a key area of development in the wellbeing of young athletes and is crucial for their safe training and successful career progression. Bone mineral density (BMD) is often used as the main (surrogate) marker for bone health, and usually peaks in early adulthood when many athletes are reaching the heights of their athletic potential (1). Ensuring young athletes reach their sporting goals without impacting their bone health can be a difficult challenge.
The adolescent years are key for the development of bone health, with approximately 90% of peak bone mass (PBM) achieved by the age of 18 years. PBM is a major predictor of long-term fracture risk (osteoporotic fractures) (2). Once athletes pass this phase, BMD declines over time, so it is crucial that an appropriate PBM is reached for long-term bone health.
What influences bone mineral density
BMD is influenced by numerous modifiable and non-modifiable risk factors (see Figure 1). Low BMD is reported to be more common in Caucasian and Asian populations as well as in post-menopausal women (2). Weight bearing sports have been shown to be a protective factor in bone health. Athletes competing in weight bearing sports have a BMD 10% higher than non-athletes, with an even higher BMD reported in athletes participating in high-impact sports (3).
Specific sports related risk factors for low BMD include Relative Energy Deficiency Syndrome (RED-S). A state of low energy availability can place athletes at risk of poor performance, low BMD and at a higher risk of osteoporotic fractures (6). If an athlete sustains a fracture, they are at risk of detrimentally impacting athletic performance, quality of life, and losing time out of training or failing to progress in their sporting careers (4).

How should we investigate bone health in the young athlete?
Basic Assessment
Assessment of bone health should begin with a comprehensive history to screen for relevant risk factors. This includes questions pertaining to nutrition, sleep, smoking, medication, exercise (with a particular emphasis on weight bearing/impact activities) and their menstrual cycle. This should be followed by a thorough physical examination and in particular a height/weight should be taken in order to calculate the patient’s body mass index (BMI).
Blood Tests
There are several blood tests that can be considered when investigating for causes of low BMD. In routine clinical practice (primary care or SEM) these may include the following blood tests prior to specialist referral:
- Full blood count
- Renal profile
- Liver function tests
- Vitamin D
- Calcium, phosphorous and magnesium
- Thyroid function tests
- Parathyroid hormone
Newer blood tests have been developed to look at markers of bone turnover (E.g. P1NP, CTX-1, Sclerostin, Osteocalcin). However, there is no definitive consensus on how they should be used in athletes and as a result their use is often restricted to either research studies or in specialist bone centres (5).
Vitamin D & Calcium – To test or not to test?
It is generally accepted that vitamin D plays a key role for the athlete in order to prevent stress fractures and muscle injury (6). The role of vitamin D supplementation and athletic performance has been debated extensively in the medical literature, however there is a lack of robust evidence to support widespread routine use (7).
Vitamin D measurement in asymptomatic patients is not routinely advised by NICE but may be considered in patients with significant risk factors for low BMD. Protocols for supplementation in vitamin D deficiency are standardised depending on plasma levels (nmol/L) and is accompanied with advice on dietary intake and sunlight recommendations.
Calcium supplementation is also not routinely recommended in the athlete and generally should only be considered if dietary intake is less than 700mg daily (or less than 1000mg a day in those with diagnoses osteoporosis) (8).
Imaging
Dual energy x-ray absorptiometry (DEXA) measures the amount of bone mineral per unit area of volume of bone tissue and is the main imaging modality used in the UK to assess BMD (9). Standard protocols measure the lumbar spine BMD (to monitor treatment) and hip BMD (to predict fracture risk). The BMD is widely measured using the T-score which is the amount of standard deviations the BMD is of a patient compared to a 30-year old healthy adult of the same sex. However, it is important to remember that in young athletes the Z-score should also be considered in order to compare scores against a healthy person of the same age and sex where we would expect the BMD to be higher (10).
| Level | Definition |
| Normal | BMD within 1 SD (+1 or −1) of the young adult mean. |
| Osteopenia | BMD between 1 and 2.5 SD below the young adult mean (−1 to −2.5 SD). |
| Osteoporosis | BMD 2.5 SD or below the young adult mean (−2.5 SD or lower). |
Table 1: World Health Organisation T scores for low bone mineral density
To help quantify fracture risk in older athletes, scoring systems such as FRAX for patients aged 40-90 (https://www.sheffield.ac.uk/FRAX/) or QFracture for patients aged 30-84 (https://qfracture.org/) may be considered. These scores are not validated for use in younger patients.

Optimising rehabilitation after a bone stress injury
The decision on when and how to return to training is a key part of the rehabilitation process after a bone stress response. The following factors should be considered (12):
- Relative rest
- The load rating of each exercise
- The total magnitude of load for the session but also the load magnitude of each exercise
- The number of loading cycles and the rest interval between sessions
- Other associated biomechanical factors that predisposed the athlete to the initial injury
Exercise at both ends of the spectrum, in terms of force/magnitude and loading rate can encourage adaption (via a sheer stress response) and promote positive bone adaptions. The bones response to loading is thought to saturate quickly so shorter sessions – spaced out with an interval of 4-8 hours, are thought to be more effective than single longer sessions (13). It is also recommended that the direction of loading and exercises should be varied and include rest periods, to help load the bone in multiple directions (14) and maximise the response.
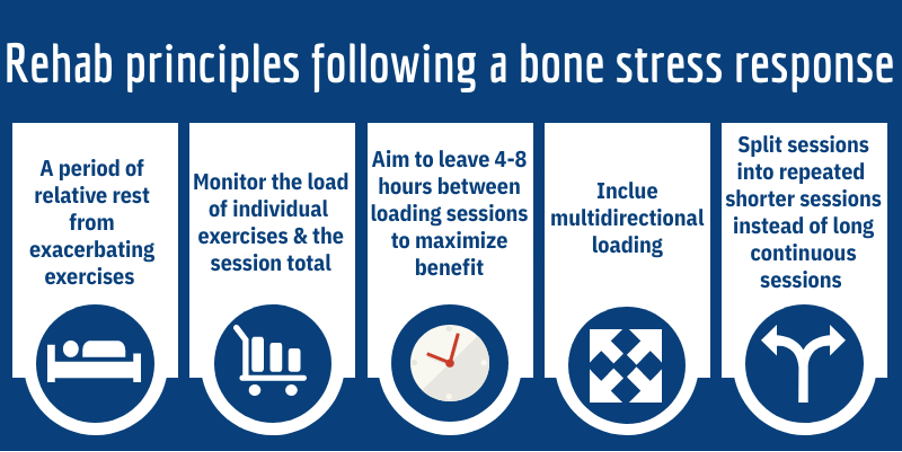
Once athletes return to training, a programme of loading three times a week is thought to be enough to encourage positive adaption and improve bone mechanical and structural properties (15).
Conclusion
- Peak bone mineral density is achieved in early adult life and these years are key in order to achieve long term adequate skeletal health.
- Low bone mineral density (BMD) is an important diagnosis not to miss in the young athlete. In those with major risk factors, a DEXA scan should be considered to investigate and potentially treat any immediate and long-term fracture risk. (Risk factors <40-years-old – major osteoporotic fractures, multiple fragility fractures or high dose glucocorticoid use)
- Athletes with low bone mineral density are at risk of fracture, loss of time to training and the potential need for prolonged rehabilitation post injury at an important development stage. This can subsequently have a significant impact on their careers, family and personal lives, all of which need to be considered.
Authors names & affiliations
Raj Amarnani
@DrRajAmar
Sports & Exercise Medicine, Imperial College Healthcare NHS Trust, London, United Kingdom
Irfan Ahmed
@Exerciseirfan
Sports & Exercise Medicine, Homerton University Hospital Foundation Trust, London, United Kingdom
Michael Giakoumis
@MickGiakoumis
Head of Medical Services and Lead Physiotherapist British Athletics Futures Program Physiotherapist at The Centre for Health and Human Performance (CHHP)
Corrine Fisher
Rheumatology Department University College London Hospitals NHS Foundation Trust, London, United Kingdom
Infographics: @Exerciseirfan
Competing Interests: Nil
References
- Goolsby MA, Boniquit N. Bone Health in Athletes. Sports Health [Internet]. 9(2):108–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27821574
- Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol [Internet]. 2006 Feb;194(2 Suppl):S3-11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16448873
- Torstveit MK, Sundgot-Borgen J. Low bone mineral density is two to three times more prevalent in non-athletic premenopausal women than in elite athletes: a comprehensive controlled study. Br J Sports Med [Internet]. 2005 May;39(5):282–7; discussion 282-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15849292
- Clay B. Bobby Clay – my osteoporosis nightmare [Internet]. Athletics Weekly. [cited 2021 Nov 5]. Available from: https://athleticsweekly.com/performance/bobby-clay-my-osteoporosis-nightmare-70422/
- Banfi G, Lombardi G, Colombini A, Lippi G. Bone metabolism markers in sports medicine. Sports Med [Internet]. 2010 Aug 1;40(8):697–714. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20632739
- Ogan D, Pritchett K. Vitamin D and the Athlete: Risks, Recommendations, and Benefits. Nutrients [Internet]. 2013 May 28;5(6):1856–68. Available from: http://www.mdpi.com/2072-6643/5/6/1856
- de la Puente Yagüe M, Collado Yurrita L, Ciudad Cabañas MJ, Cuadrado Cenzual MA. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients [Internet]. 2020 Feb 23;12(2). Available from: http://www.ncbi.nlm.nih.gov/pubmed/32102188
- NICE. Vitamin D deficiency in adults [Internet]. 2021 [cited 2021 Nov 1]. Available from: https://cks.nice.org.uk/topics/vitamin-d-deficiency-in-adults/
- Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J [Internet]. 2007 Aug;83(982):509–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17675543
- Carey JJ, Delaney MF, Love TE, Richmond BJ, Cromer BA, Miller PD, et al. DXA-generated Z-scores and T-scores may differ substantially and significantly in young adults. J Clin Densitom [Internet]. 10(4):351–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17662630
- NICE. Osteoporosis: assessing the risk of fragility fracture [Internet]. 2017 [cited 2021 Nov 1]. Available from: https://www.nice.org.uk/guidance/cg146
- Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone [Internet]. 2002 May;30(5):781–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11996920
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am [Internet]. 1984 Mar;66(3):397–402. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6699056
- Judex S, Zernicke RF. High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J Appl Physiol [Internet]. 2000 Jun 1;88(6):2183–91. Available from: https://www.physiology.org/doi/10.1152/jappl.2000.88.6.2183
- Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res [Internet]. 2005 May;20(5):809–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15824854