In the last few weeks I have been discussing criteria that prescribers may consider in deciding whether to introduce a therapeutic intervention. They include:
- the minimal clinically important difference (MCID), a measure of how large a change in the patient’s condition needs to be to justify using an intervention, assuming a good benefit to harm balance and cost-effectiveness;
- the minimal important difference (MID), a similar measure, omission of the word “clinically” being intended to focus attention on the patient rather than the clinician;
- the importance of the size of the difference, which, if achieved, is not always important to the patient, but may be important to others, such as family or carers, or even the clinician;
- the patient acceptable symptom state (PASS), a much less widely used term, intended to focus on “the highest level of symptom beyond which patients consider themselves well”.
At the end of these explorations I came to the conclusion that it would be helpful to introduce two new concepts to clarify the ideas that the various terms imply: minimum desirable benefits and maximum acceptable harms. These terms imply that what is acceptable to the patient (or family or carers) in terms of beneficial outcomes may not be what the patient actually wants, and that whatever the patient wants in the way of benefit must be tempered by the realization that harms can occur and that what matters is not either the desire to achieve benefit nor the hope of avoiding harms, but a combination of the two, commonly expressed as the benefit to harm balance, which has three components:
- the probability of benefit from using the treatment;
- the probability of harm from using the treatment;
- the probability of harm from not using the treatment.
The harm side of the balance is a composite that reflects the difference in risks from the two possible sources of harm.
In considering whether individual benefit is to be gained, judgment in the first place relies on the results of population studies, combined with information about characteristics of the patient that may predict whether and to what extent benefit is likely (e.g. age, sex, renal and hepatic function, concurrent medications that might interact with the proposed intervention, previous experience of other interventions, and in a few cases genetic factors).
If an intervention is introduced, one then has the opportunity to determine whether the desired outcome has been achieved, by monitoring. Here two forms of information are available. First, objective measures of outcomes: Has the blood pressure fallen satisfactorily? Has the haemoglobin concentration risen? Is the cellulitis regressing?
Secondly, and particularly when objective observations are not possible, one relies on the patient’s report of the extent to which unwanted symptoms have been reduced or eliminated: Is there less anxiety? Is the pain less severe? Has the anorexia abated, the appetite improved?
In assessing improvement in symptoms the term “patient reported outcomes” is often used. The earliest instance of this term listed in PubMed dates from 1976, but there is nothing after that until 1992 (Figure 1). The related term “patient reported outcome measures” first appeared in PubMed in 2003, although earlier instances can be found elsewhere, going back to at least 1994. Of about 15 000 items listed in PubMed, about 250 are specified in their titles as being systematic reviews of patient reported outcomes; the first, on the effect of growth hormone on cognitive functions, was published in 2005.
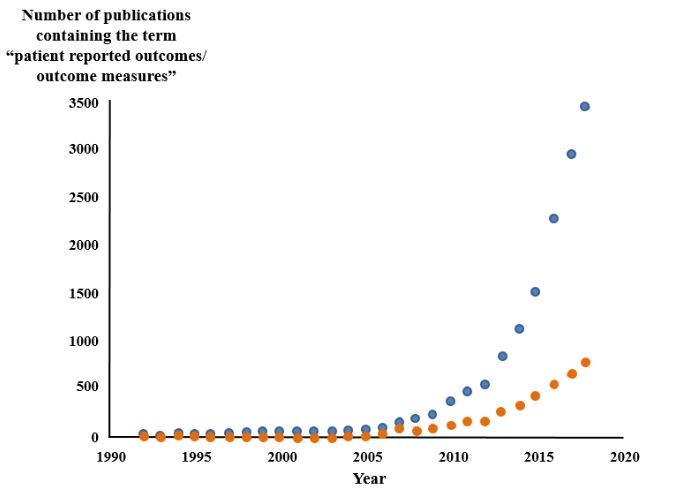
Figure 1. The numbers of papers containing the term “patient reported outcomes/outcome measures” since 1992 (source PubMed); one paper in 1976 has been omitted; blue symbols text words, orange symbols titles; the term has become increasingly popular since about 2010
Other terms that have been affixed to “patient reported” include: outcome instrument, physician-patient discussion, purpose of visit, problem status, experience, discussion, satisfaction, change, [severity of] symptoms, outcomes difference, and questionnaires.
In contrast “clinician reported outcomes” yields only 57 hits, “carer/caregiver reported outcomes” 25, and “family reported outcomes” a mere nine; one paper referred to “family caregiver-reported outcomes”. While it appears that there is little about healthcare that a patient cannot report, what others might report seems to be of little interest.
 Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.
