Author: Georgios Kakavas PT OMT PhD
The purpose of this blog is to explain the phenomenon of arthrogenic muscle inhibition (AMI) after anterior cruciate ligament (ACL) reconstruction. It also aims to determine reported mechanisms behind AMI in patients with ACL injuries, or following ACL reconstruction (ACLR), and to propose strategies effective in improving quadriceps activation.
Persistent quadriceps weakness is a problematic sequel of ACLR and the sports medicine community needs a consensus for its solution. A further purpose of this blog is to summarize neuroplastic changes after ACLR.
Introduction
ACL injury is one of the most common sports-related injuries, and its incidence has been increasing at all levels of competition in recent years. In the United States alone, the rates of ACL reconstruction (ACLR) have increased significantly over the last 12 years from 10.36 to 18.06 and from 22.58 to 25.42 per 100,000 person-years for females and males, respectively (1). Given the rise in ACL injuries and revision rates, there has been an increased interest in understanding ACLR graft choices to improve outcomes, decrease morbidity, and lower revision rates.
Observations from sports medicine professionals evaluating athletes post-ACLR surgery reveal a significant issue: the muscles around the knee often show faint or no contraction (2). This is a manifestation of AMI, a phenomenon where otherwise healthy muscles become reflexively inhibited following a joint injury. Unfortunately, AMI can occur regardless of sex, age, or the injured joint, and it acts as a significant barrier in rehabilitation, hindering muscle strength and functional recovery if left untreated. This is shown in Figure 1.
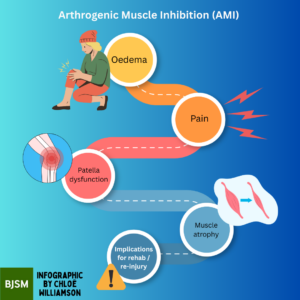
Figure 1: AMI flowchart. This flowchart illustrates the complex process of AMI, from the initial injury to the joint to the reflexive inhibition of the surrounding musculature. Understanding this process is key to developing effective rehabilitation strategies.
AMI and knee muscles’ neural pathway
Marked weakness of the quadriceps muscle group is present after knee joint injury from a presynaptic reflex inhibition of the musculature surrounding the knee joint. AMI persists after an ACL injury and during rehabilitation after ACLR, while deficits in central neural activation persist after ACLR and beyond return to sport (3). The muscle inhibition responsible for these observations is likely reflexive and these observations occur primarily during early phases of recovery, plausibly from changes in joint function secondary to a combination of tissue damage, joint laxity, effusion, pain, and inflammation. It has been argued from other researchers that these changes disrupt the normal neural signalling between joint receptors and the central nervous system, altering the sensory information transmitted to the spinal cord and brain (4).
Despite extensive clinical research, return to sport rates after ACLR is suboptimal. In an updated systematic review by Ardern et al., [69 articles; n = 7,556 athletes (66% male); average age: 25 ± 3.2 years], the authors found that only 65% of athletes returned to their preinjury sport level after ACLR; of those who previously participated in competitive sports, only 55% returned to the competitive level after ACLR (5).
Understanding the pathophysiology of AMI is crucial for developing targeted therapeutic interventions. Muscle force generation is a product of both motor unit rate coding and quantity of motor unit recruitment, with an inverse relationship between motor unit rate coding and motor unit recruitment threshold. AMI significantly impacts recovery after ACLR, and appropriate physiotherapy should address AMI after ACL injury or reconstruction. Several studies like Bertrand Sonnery-Cottet et al., report an association between hamstring dysfunction and dyskinesia with quadriceps weakness in AMI (6). This has been attributed to the flexion reflex spinal pathway, which produces a pattern of flexor facilitation and extensor inhibition. Greater hamstring co-activation is associated with significantly worse knee function (7).
Brain connection of AMI
Neuroplasticity, the central nervous system’s ability to adapt in response to environmental or anatomical factors, plays a crucial role in recovery. These adaptations may involve changes in cognitive strategies, recruitment of different neural circuits, or amplification or reduction of involvement of certain connections or brain areas (8). By utilizing the concepts of neuroplasticity, rehabilitation experts can tackle the intricate interactions of physical and neurocognitive elements that affect results following ACL injuries.
Neurocognitive tasks measuring reaction time, processing speed, visual memory, and verbal memory are well established in the neuropsychology literature as indirect measures of cerebral performance (add citation). The individual’s situational awareness, arousal, and attentional resources may influence these areas of neurocognitive function, affecting the complex integration of vestibular, visual, and somatosensory information needed for neuromuscular control (9).
Following an ACLR, the central nervous system may increase its reliance on alternative sensory sources, such as visual feedback and spatial awareness. This shift in sensory processing may contribute to the persistence of AMI. Post ACLR athletes exhibited increased activation in the posterior inferior temporal gyrus (visual processing), pre-supplementary motor area (motor planning), and secondary somatosensory area (pain and sensory processing), suggesting a reorganization of neural pathways in response to the injury (10).
Key messages
- The ACL has more than biomechanics role in the knee stability; the ACL injury and rehabilitation process must talking that as consideration.
- Rehabilitative interventions help to restore dynamic knee stability after ACLR. Future research must focus to optimize interventions to address lingering motor control deficits despite current recommended clinical practice.
- AMI contributes to the characteristic muscle weakness, activation failure, and atrophy observed in patients recovering from joint injuries.
- Understanding the implications of AMI for rehabilitation post-ACLR is crucial for developing effective treatment strategies.
References
- Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. doi:10.1123/jsr.9.2.135
- Deandrade JR, Grant C, Dixon AS. Joint distension and reflex muscle inhibition in the knee. J Bone Joint Surg Am. 1965;47(2): 313–322. PubMed ID: 14261807 doi:10.2106/00004623- 196547020-00008
- McPherson AL, Schilaty ND, Anderson S,Nagai T and Bates NA (2023) Arthrogenic muscle inhibition after anterior cruciate ligament injury: Injured and uninjured limb recovery over time.Front. Sports Act. Living 5:1143376. doi: 10.3389/fspor.2023.1143376
- Lepley AS Gribble PA Thomas AC, et al. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: A 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25:828-39.
- Ardern CL, Taylor NF, Feller JA, et alFifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factorsBritish Journal of Sports Medicine 2014;48:1543-1552.
- Norte G, Rush J, Sherman D. Arthrogenic muscle inhibition: best evidence, mechanisms, and theory for treating the unseen in clinical rehabilitation. Journal of sport rehabilitation. 2021 Dec 9;31(6):717-35
- Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10(6): 329–335. PubMed ID: 6897495 doi:10.1177/036354658201000601
- Kakavas G, Malliaropoulos N, Pruna R, Traster D, Bikos G, Maffulli N. Neuroplasticity and Anterior Cruciate Ligament Injury. Indian J Orthop. 2020 Jan 31;54(3):275-280. doi: 10.1007/s43465-020-00045-2. PMID: 32399146; PMCID: PMC7205971.
- Buckthorpe M, La Rosa G, Villa FD. RESTORING KNEE EXTENSOR STRENGTH AFTER ANTERIOR CRUCIATE LIGAMENT RECONSTRUCTION: A CLINICAL COMMENTARY. Int J Sports Phys Ther. 2019 Feb;14(1):159-172. PMID: 30746302; PMCID: PMC6350662.
- Dustin R. Grooms, Stephen J. Page, Deborah S. Nichols-Larsen, Ajit M.W. Chaudhari, Susan E. White, and James A. Onate Neuroplasticity Associated With Anterior Cruciate Ligament Reconstruction Journal of Orthopaedic & Sports Physical Therapy 2017 47:3, 180-189