Greater Trochanteric Pain Syndrome (GTPS) is a common cause of lateral hip pain seen in the MSK clinic and can have a significant impact on patient’s function, and ability to exercise pain free. Understanding which lateral hip soft tissue structures are affected can help to design effective recovery and exercise strategies. We also discuss indications for imaging and when it can be used to guide injection, shockwave or pain-relieving procedures.
Diagnosing GTPS
GTPS is a condition diagnosed based on the presence of typical clinical symptoms affecting the lateral hip structures (1).
- Pain with palpation
- Pain that is worse with adducted hip positions,
- Pain with weightbearing and resisted abduction
- The absence of other hip conditions (OA/FAI)
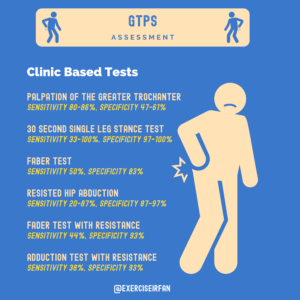
Figure 1: The sensitivities and specificities of clinic-based tests used in the assessment of GTPS
GTPS symptoms are often diffuse and travel to other structures. Symptoms may radiate down the thigh to the knee, to the gluteal region, or superior to the hip depending on which structures are impacted. Considering the results of these clinical tests in the context of the presenting complaint and risk factors is key to diagnosis.
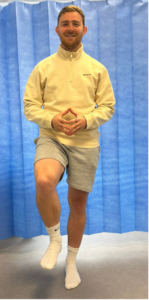 Figure 2: Single Leg Stand Test – SLS test
Figure 2: Single Leg Stand Test – SLS test
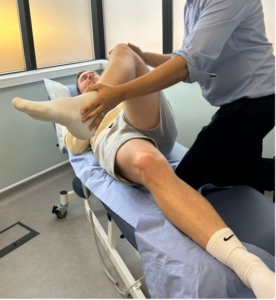 Figure 3: Flexion Adduction External rotation – FADER test
Figure 3: Flexion Adduction External rotation – FADER test
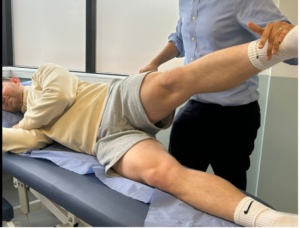 Figure 4: Resisted hip abduction – in side lying
Figure 4: Resisted hip abduction – in side lying
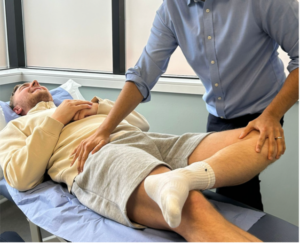 Figure 5: FABER test
Figure 5: FABER test
Understanding the burden of GTPS
- Cause of 10-20% of hip pain presenting to primary care (2,3)
- Incidence of GTPS is 1.8 patients per 1000 per year (2)
- Prevalence highest amongst 40–60-year-olds – but can affect any age (3)
- 1 in 4 women over 50 have been affected (4)
- The female: male ratio for GTPS is approximately 4:1 (4)
- Pain can be exacerbated by prolonged sitting, lying on affected side, climbing stairs and by high-intensity training
- Mean duration of symptoms prior to treatment is 7.1 weeks – 4.4 years (4-6)
A chronic overload of the soft tissues
GTPS is thought to be due to excessive or progressive overload of the soft tissue structures of the lateral hip. It is almost exclusively seen as a chronic condition, with acute or isolated inflammatory presentations in the minority. When working up patients in the MSK clinic it is important to be aware of the following risk factors:
- Age (Middle age status 40-60)
- Metabolic disrepair or metabolic medical conditions – (E.g. diabetes, Rheumatoid arthritis, high BMI)
- Overload history
- Friction symptoms during provoking movements.
- Sex (female sex is associated with an increased risk of GTPS)
- Biomechanical risk factors (leg length discrepancy, scoliosis)

Figure 6: Intrinsic v Extrinsic risk factors for GTPS
The big two GTPS pathologies; tears and tendinopathy!
By far the most common causes of GTPS are tendinopathy and partial thickness tears of the glute tendons. Understanding the underlying causes for these two conditions is key in aiding diagnosis and developing a treatment plan. Patients often attend with ideas about the diagnosis and that may be due to “hip bursitis” or “trochanteric bursitis”. Recent imaging and histological studies however have shown that where present associated bursae are almost exclusively a friction phenomenon with acute bursitis, rarely seen on histological or clinical presentations (7).
A practical approach to GTPS; unpicking superficial v deep soft tissue causes
The lateral hip soft tissue structures can be divided into the:
- Superficial layer – Gluteus maximus and tensor fasciae latae (TFL)
- Deep layer – Gluteus medius and gluteus minimus
Each of these layers have their own function, biomechanical properties, and respond differently to load. Recent studies have shown that ITB and glute max (superficial layers), may have additional roles in absorbing elastic energy and improving the economy of movement, whilst the deep layer initiates and generates forces in hip abduction. Forces of up to 6-8 times body weight have been recorded during walking/jogging across the literature (8).
| TFL / ITB / Gluteus Maximus | Gluteus Medius / Gluteus Minimus | TFL / ITB / Gluteus Maximus | Gluteus Medius / Gluteus Minimus | |
| Lateral hip layer | Superficial | Deep | Superficial | Deep |
| Activity | Walking | Walking | Running | Running |
| Typical forces | Compression forces negligible | Compressive forces
++++ |
||
| Function | ITB acts as a strut in frontal plane and energy storage | Hip stabilisation Prevent Trendelenburg gait | ITB acts as a strut in frontal plane and energy storage | Hip stabilisation
Prevent Trendelenburg gait |
| Most active during which phase of movement | ITB/TFL
-Early swing & -Support phase Gluteus Maximus -Trunk stabilisation |
ITB/TFL
-Early swing -Beginning of stance phase -End of swing phase Gluteus Maximus -Initial contact phase – stops the hip going into flexion in impact (concentric) -Late swing phase – slows down hip flexion to get ready for contact (eccentric) |
||
| Energy storage | Yes – ITB | No | Yes – ITB
Increasing elastic properties at higher speeds |
No |
Table 1: Explanation of the forces, energy storage, and function at each layer and muscle (9-11)
The pathophysiology of GTPS
When working up patients in the MSK clinic, it is important to ask about key risk factors that can influence rehab and response to treatment. These can be divided into medical, biomechanical, and degenerative causes.
| Medical | Biomechanical | Degenerative/ tendinopathy |
| – Obesity (High BMI)
– Enthesopathy secondary to medical conditions – Diabetes |
– Deconditioning
– Weak glutes – Pelvic tilt – External coxa saltans (snapping hip) |
– Post trauma
– Tendinopathy features |
Table 2: key risk factors that can influence GTPS rehab and treatment response (4, 6,12)
Whilst GTPS is predominantly a clinical diagnosis, imaging can be used to rule out significant pathology of the hip joint (OA), or to confirm the diagnosis in recalcitrant or persistent cases. X-rays can be used to assess hip OA and is the most widely available imaging modality.
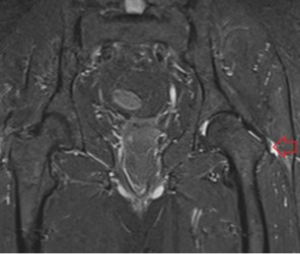
Figure 7: MRI scan
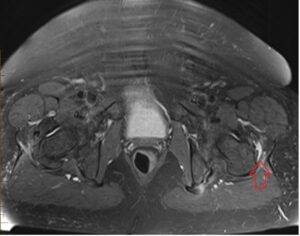
Figure 8: MRI scan
Imaging the soft tissue of the lateral hip
Point of care Ultrasound and MRI both have, good sensitivity and specificity for assessing soft tissue structure of the lateral hip. Ultrasound has the added benefit of being easily accessible, allows for dynamic examination, (snapping phenomena) and also allows you to assess soft tissue structure in clinic
Gluteus Medius tears and tendinopathy are most commonly picked up by point of care ultrasound or MRI. In the absence of red flags serious pathology of the underlying hip joints is rarely found. Comparing the two, ultrasound is the cheaper, quicker and more widely accessible mode of imaging.
| Finding | MRI Reporting Rates (%) | USS Reporting Rates (%) |
| Gluteus medius tear (partial or full thickness) | 46 – 63 | 1-33 |
| Gluteus medius tendinopathy | 37-73 | 34-71 |
| Gluteus minimus tendinopathy | 29 | 13-23 |
| Trochanteric bursitis | 8 – 40 | 8-75 |
| Thickened ITB | – | 0-29 |
| Partial ITB tear | – | 1 |
| Enthesopathy | – | 22 |
| Avascular necrosis of femoral head | 4 | – |
Table 3: imaging findings and MRI vs USS reporting rates (13-16)
Ultrasound and MRI both provide soft tissue detail and can be used to image the soft tissues of the lateral hip. There is however a significant false positive rate for imaging findings, and many imaging findings for tendinopathy can be seen in asymptomatic patients (13,14,17). A study by Grimaldi et al. (18) found that 31% of participants with MRI findings suggestive of gluteal tendinopathy had no clinical symptoms of GTPS. It is important to put any imaging findings in the context of the pre-test findings (18,19).
Common MRI findings seen on Imaging – GTPS (example cases)
| MRI Finding | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| High signal superior to greater trochanter | 73 | 95 | 91 | |
| Tendon elongation in gluteus medius muscle | 53 | 86 | 80 | |
| Tendon discontinuity | 27 | 100 | 85 | |
| High signal lateral to greater trochanter | 53 | 80 | 74 | |
| Atrophy of abductor muscles | 40 | 86 | 77 | |
Overall MRI diagnosis of tendon tear defined by triad of:
|
93 | 92 | 91 |
Table 4: common MRI findings seen on imaging for GTPS – example cases (20).
One shortfall when it comes to treatment is expecting a rapid resolution in symptoms with almost 50% experiencing ongoing pain at one year (21). We know from imaging studies that symptoms are typically driven by tendinopathic changes at the glute tendons which is linked to metabolic health, and metabolic dysregulation. GTPS is commonly seen in peri-menopausal and middle Aged patient with other medial risk factors (21-23). Therefore, arguably the most important interventions involve improving metabolic health, education around tendon loading, lifestyle measures and identifying reversible risk factors. (Figure 5). Treatment in MSK settings should involve discussions around:
- Exercise prescription
- Weight management
- Social prescribing
- Pain management options
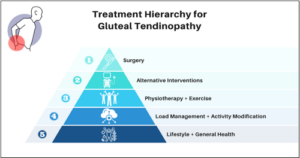
Figure 9: treatment hierarchy for gluteal tendinopathy.
Education regarding load management can offer some quick wins to help alleviate symptoms by reducing compressive load through the tendons, or snapping of the tendons with external coxa saltans. This has been shown to offer significant change in pain and function in as little as 8-12 weeks, irrespective of the exercise programme (21, 24). Examples include:
- Sleep – on your back, or the unaffected side with a pillow between the knees or purchasing a mattress topper.
- Sitting – look to reduce time spent in cross-legged positions or in positions of deep hip flexion as it can increase compressive load.
- Track Step Count – adequate load management is key and monitoring steps is a useful method to help prevent overloading the tendon.
- Terrain – for our athletic population, considering a temporary reduction with incline or cambered terrain during running will help.
It is important to inform the patient that none of these positions are inherently bad. Yet, temporarily reducing them may offer sufficient relief, especially in the earlier phases with reactive tendinopathy subtypes. This is advantageous as it may facilitate a return to meaningful activities and thus improve quality of life, especially when different exercise regimes have not illustrated superior outcomes (21).
Exercise
Exercise forms the mainstay of initial treatment for lower limb tendinopathies with the rationale designed to restore capacity towards the tendon (25). Individuals with GTPS have demonstrated hip abduction strength deficits accompanied with altered biomechanics whilst walking, with increased hip adduction and contralateral pelvic drop (9). Although unable to prove whether this is association or causation, we can hypothesise these deficits may influence symptoms due to greater compressive load through the tendons and this is something we can look to target during rehabilitation.
Exercise combined with education appears to be superior versus a wait and see approach at short and long-term outcomes (24). Yet, the type of exercise doesn’t appear to have much of an influence on success and this supports the notion that improved outcomes might not solely be due to physiological changes, but instead better self-efficacy (24, 26, 27). Additionally, those with more advanced GTPS have demonstrated higher BMIs, psychological distress and reduced activity (28). Therefore, encouraging generic forms of physical activity such as aquatic therapy, cycling or walking, particularly in a social/supportive environment may help address these variables. For specific exercises, a stepped approach which looks to progressively load the tendon at high intensity without provoking symptoms is essential for tendinopathies, with a particular emphasis towards the frontal plane in GTPS (29, 31). Some examples are illustrated in Figure 6.
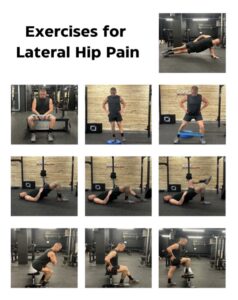
Figure 10: exercises for lateral hip pain.
Corticosteroid Injections (CSI)
Once considered the first line treatment for GTPS, injections are now encouraged to be used more judiciously. CSI offer a significant reduction in pain intensity with 75% of patients reporting a significant improvement in symptoms at one month, but these effects gradually decreased with time (31). More recently, a single CSI outperformed a wait and see approach at two months and one year but was less effective compared to education and exercise for success on global rating of change. The risk of repetitive injections must be balanced against tendon health, and higher doses may impact tendon health (homeostasis) (32). Especially as isolated bursitis in the absence of tendinopathic changes to the hip abductors is extremely rare (33).
Other Interventions
There is a paucity of research surrounding taping for GTPS. One study compared dynamic taping versus sham and found both offered improvements in pain, whilst the former influenced hip biomechanics whilst walking (34). Further studies are needed to ascertain the physiological effects of taping on this condition.
KEY GTPS TREATMENTS (3):
- Weight loss
- NSAIDs
- Ice
- Taping
- Rest
- Targeted physiotherapy (tailored to individual but focusing on gluteal strength and control followed by hip abductor strength)
- Load modification (sleeping with pillows in between legs to reduce compression on gluteal tendons)
- Biomechanical optimisation (avoid excessive hip adduction positions – i.e. crossing legs or excessive ITB stretching)
- Shockwave therapy (ESWT)
- Therapeutic ultrasound
- Corticosteroid injections
- Platelet rich plasma (PRP) injections
- Surgical intervention (only after failed conservative management – may involve lengthening/release of ITB and fascia lata or gluteal tendon repair)
LEAP trial – key findings (24):
The LEAP trial was a randomised clinical trial conducted in Australia which aimed to compare the effects of an education about tendon load management with a specific exercise programme of 14 sessions over 8 weeks, a single corticosteroid injection and a wait-and-see approach on pain and general improvement in people aged between 35-70 with gluteal tendinopathy (lateral hip pain for > 3 months). Patients were asked to report a change in hip condition and pain intensity during the last week at eight weeks, followed by a longer follow-up at 52 weeks.
Outcomes
- 189/204 participants completed the 52-week follow-up
- Education with exercise reported less pain than corticosteroid injections
- Education with exercise and corticosteroid injection use had higher rates of global improvement and lower pain scores than wait and see patients at eight weeks
- At 52 weeks, education with exercise had better global improvement than corticosteroid injections, but there was no difference in pain intensity
- Education with exercise is supported as an effective management plan for gluteal tendinopathy based on these results
Injection Therapies for GTPS
Injection therapies can play an important role in the treatment of greater trochanteric pain syndrome, especially when other conservative management practices strategies have failed to achieve adequate symptom control.
- Corticosteroid Injections
Can be effective in providing pain relief and functional improvements, but repetitive injection may impact long term tendon health. The total steroid burden and frequency of injections should be discussed as part of a shared decision making process.
- New orthobiologic injection approaches – Platelet Rich Plasma (PRP)
Studies have shown that these may be more effective and provide more long-term pain relief (Table 5). PRP injection have the benefit of avoiding steroid burden and can be combined with or without dry needling procedures. The aim of these injections is to improve tendon health and provide a regenerative approach to treatment.
| Authors | Type of Study | Comparison of Treatments | Outcomes |
| Fitzpatrick et al. (35) | Double blind randomised control trial with 2 year follow up | PRP injection vs. corticosteroid injection | A single PRP injection resulted in greater and longer lasting improvements in pain and function than a single corticosteroid injection.
The effects of the PRP were sustained at 2 years, whereas the improvements from the corticosteroid injection peaked at 6 weeks and were not maintained beyond 24 weeks. |
| Migliorini et al. (36) | Systematic review and meta-analysis | PRP injection vs. corticosteroid injection | PRP injections provide much longer and more sustained pain relief when compared to corticosteroid injections.
|
Table 5: Summary of notable studies comparing PRP injections to corticosteroid injections
Shockwave therapy
Extracorporeal shock wave therapy (ESWT) has been found to be a suitable alternative treatment option for GTPS by several studies (37). Seo et al found that low-energy ESWT can be an effective treatment for chronic GTPS but over time the effects decrease (38).
Ramon et al. (37) found that when ESWT is completed in conjunction with a specific exercise programme, it is effective for GTPS, with a high success rate of 86.8% that was maintained until the end of the follow up period (6 months)
Pressure waves from ESWT can deliver a mechanical force to the tendons, which stimulates blood flow (increasing rates of healing). Shockwaves can mimic forces that occur during trauma and can promote healing and regeneration (39).
| Authors | Type of Study | Comparison of Treatments | Outcomes |
| Heaver et al. (40) | Randomised control trial | Focussed shockwave therapy vs. ultrasound guided corticosteroid injection | At 12 months, the shockwave group had significantly better pain and functional scores compared to the corticosteroid injection group. |
| Ramon et al. (37) | Randomised control trial | Shockwave therapy and exercise vs. sham shockwave therapy and exercise | Significantly better pain and functional outcomes in the shockwave group through 6 months of follow up. However, more studies are needed. |
| Harding et al. (41) | Systematic review and meta-analysis | Shockwave therapy vs control treatments | There is only low strength evidence that shockwave can lead to greater clinical improvements over time when compared to control treatments. |
Table 6: Summary of notable studies assessing the use of extracorporeal shockwave therapy
Fascial plane block
Ultrasound guidance has been used to help with targeting joints, muscles, nerves and tendons for procedures. For the hip specifically, ultrasound guidance has become increasingly common practice for procedures involving the lateral and posterior hip (42). The superior gluteal nerve innervates the superior femoral neck, postero-lateral hip joint capsule and the lateral hip muscles (gluteus medius, gluteus minimus and tensor fasciae latae). As the SGN innervates the lateral hip muscles, it is a potential block target for patients presenting with GTPS.
Fascial plane blocks are a regional anaesthesia technique where the plane between two fascial layers is targeted by anaesthetic, which spreads to nerves within this plane and the adjacent tissues.
Ferreira-Dos-Santos et al. (42) found that whilst the fascial plane block is able to block the SGN without involving the sciatic or inferior gluteal nerves, further randomised controlled clinical trials are necessary to assess the effects of fasical plane blocks of the SGN in moderate-to-severe and chronic GTPS.
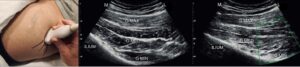
Figure 11: Facial plane block from Ferriera-Dos Santos et al. (42)
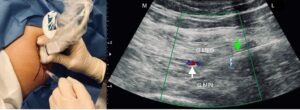
Figure 12: Fascial plane block from Ferriera-Dos Santos et al. (42)
Conclusion
- GTPS is a common cause of lateral hip pain in working age patients, associated with chronic MSK pain and restriction to exercise.
- Understanding the function and structural properties of the “Deep V Superficial” soft tissues layers of the lateral hip can help to plan rehab and aid clinical reasoning around treatment options.
- Point of care ultrasound, is increasingly available in MSK clinics and can be used to assess the gluteal tendons, and plan injection therapy for recalcitrant cases
- Treatment options for GTPS, should start with patient education, optimising metabolic risk factors and exercise. Shockwave therapy, injection options or pain interventions can be considered on a personalised basis, taking into account symptom severity and duration.
Authors:
Lynsey Abbey Joslin, Dr Ryan Linn, Dr Imran Lasker, Charlie Clements, Dr Guilherme Ferreira-Dos-Santos, MD, Mr Tom Edgeley Dr Dr Jeffrey Peng, Dr Irfan Ahmed.
Lynsey Abbey Joslin
Medical Student, University College London (UCL)
Dr Ryan Linn
Foundation Year 1 Doctor, University Hospitals of North Midlands
Twitter: @Ryan_Linn_
Dr Imran Lasker
Consultant Radiologist with Special Interest in MSK, Mid & South Essex Foundation Trust
Twitter: @DocLasker
Links to MSK/MRI courses: imranlasker.com, radiologyseminars.com. emergencyimaging.co.uk
Charlie Clements
Sirona Care & Health, First Contact Physiotherapist
Twitter @ClementsCharl96
Dr Guilherme Ferreira-Dos-Santos, MD
Senior Specialist and Responsible Clinical Lead for the Education and Training Excellence Center in Pain Medicine, accredited by the European Society of Regional Anesthesia and Pain Therapy
Affilitation: Division of Pain Medicine, Department of Anesthesiology, Reanimation, and Pain Medicine, Hospital Clínic de Barcelona, University of Barcelona. Barcelona, Spain.
Dr Jeffrey Peng MD, CAQSM
Sports Medicine Physician
Clinical Assistant Professor (Affiliated); Stanford University School of Medicine, Department of Medicine, Division of Primary Care & Population Health
Twitter: @JeffreyPengMD
YouTube: youtube.com/c/JeffreyPengMD
Website: www.JeffreyPengMD.com
Mr Tom Edgeley
Health advisor, BUPA St Albans
Dr Irfan Ahmed
Consultant in Musculoskeletal, Sport & Exercise Medicine
twitter @exerciseirfan
References
- Williams BS, Cohen SP. Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg. 2009;108(5):1662-1670. doi:10.1213/ane.0b013e31819d6562
- Lievense A, Bierma-Zeinstra S, Schouten B, Bohnen A, Verhaar J, Koes B. Prognosis of trochanteric pain in primary care. Br J Gen Pract. 2005;55(512):199-204.
- Speers CJ, Bhogal GS. Greater trochanteric pain syndrome: a review of diagnosis and management in general practice. Br J Gen Pract. 2017;67(663):479-480. doi:10.3399/bjgp17X693041
- Segal NA, Felson DT, Torner JC, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil. 2007;88(8):988-992. doi:10.1016/j.apmr.2007.04.014
- Livingston JI, Deprey SM, Hensley CP. DIFFERENTIAL DIAGNOSTIC PROCESS AND CLINICAL DECISION MAKING IN A YOUNG ADULT FEMALE WITH LATERAL HIP PAIN: A CASE REPORT. Int J Sports Phys Ther. 2015;10(5):712-722.
- Pumarejo Gomez L, Li DD, Childress JM. Greater Trochanteric Pain Syndrome (Greater Trochanteric Bursitis). In: StatPearls. Treasure Island (FL): StatPearls Publishing; February 25, 2024.
-
Long SS, Surrey DE, Nazarian LN. Sonography of greater trochanteric pain syndrome and the rarity of primary bursitis. AJR Am J Roentgenol. 2013 Nov;201(5):1083-6. doi: 10.2214/AJR.12.10038. PMID: 24147479.
- Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829
- Jeong DE, Lee SK, Kim K. Comparison of the activity of the gluteus medius according to the angles of inclination of a treadmill with vertical load. J Phys Ther Sci. 2014;26(2):251-253. doi:10.1589/jpts.26.251.
- Eng CM, Arnold AS, Lieberman DE, Biewener AA. The capacity of the human iliotibial band to store elastic energy during running. J Biomech. 2015;48(12):3341-3348. doi:10.1016/j.jbiomech.2015.06.017
- Lieberman DE, Raichlen DA, Pontzer H, Bramble DM, Cutright-Smith E. The human gluteus maximus and its role in running. J Exp Biol. 2006;209(Pt 11):2143-2155. doi:10.1242/jeb.02255
- Kadar A, Itzikovitch R, Warschawski Y, Morgan S, Shemesh S. Diabetes Mellitus Is a Possible Risk Factor for the Development of Trochanteric Bursitis-A Large-Scale Population-Based Study. J Clin Med. 2023 Sep 24;12(19):6174. doi: 10.3390/jcm12196174. PMID: 37834819; PMCID: PMC10573166.
- Kingzett-Taylor A, Tirman PF, Feller J, et al. Tendinosis and tears of gluteus medius and minimus muscles as a cause of hip pain: MR imaging findings. AJR Am J Roentgenol. 1999;173(4):1123-1126. doi:10.2214/ajr.173.4.10511191
- Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum. 2001;44(9):2138-2145. doi:10.1002/1529-0131(200109)44:9<2138::AID-ART367>3.0.CO;2-M
- Long SS, Surrey DE, Nazarian LN. Sonography of greater trochanteric pain syndrome and the rarity of primary bursitis. AJR Am J Roentgenol. 2013;201(5):1083-1086. doi:10.2214/AJR.12.10038
- Hilligsøe M, Rathleff MS, Olesen JL. Ultrasound Definitions and Findings in Greater Trochanteric Pain Syndrome: A Systematic Review. Ultrasound Med Biol. 2020;46(7):1584-1598. doi:10.1016/j.ultrasmedbio.2020.03.008
- Blankenbaker DG, Ullrick SR, Davis KW, De Smet AA, Haaland B, Fine JP. Correlation of MRI findings with clinical findings of trochanteric pain syndrome. Skeletal Radiol. 2008;37(10):903-909. doi:10.1007/s00256-008-0514-8
- Grimaldi A, Mellor R, Nicolson P, Hodges P, Bennell K, Vicenzino B. Utility of clinical tests to diagnose MRI-confirmed gluteal tendinopathy in patients presenting with lateral hip pain. Br J Sports Med. 2017;51(6):519-524. doi:10.1136/bjsports-2016-096175
- Westacott DJ, Minns JI, Foguet P. The diagnostic accuracy of magnetic resonance imaging and ultrasonography in gluteal tendon tears–a systematic review. Hip Int. 2011;21(6):637-645. doi:10.5301/HIP.2011.8759
- Cvitanic O, Henzie G, Skezas N, Lyons J, Minter J. MRI diagnosis of tears of the hip abductor tendons (gluteus medius and gluteus minimus). AJR Am J Roentgenol. 2004;182(1):137-143. doi:10.2214/ajr.182.1.1820137
- Ganderton C, Semciw A, Cook J, Moreira E, Pizzari T. Gluteal Loading Versus Sham Exercises to Improve Pain and Dysfunction in Postmenopausal Women with Greater Trochanteric Pain Syndrome: A Randomized Controlled Trial. J Womens Health (Larchmt). 2018;27(6):815-829. doi:10.1089/jwh.2017.6729
- Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford). 2013;52(4):599-608. doi:10.1093/rheumatology/kes395
- Pianka MA, Serino J, DeFroda SF, Bodendorfer BM. Greater trochanteric pain syndrome: Evaluation and management of a wide spectrum of pathology. SAGE Open Med. 2021;9:20503121211022582. Published 2021 Jun 3. doi:10.1177/20503121211022582
- Mellor R, Bennell K, Grimaldi A, et al. Education plus exercise versus corticosteroid injection use versus a wait and see approach on global outcome and pain from gluteal tendinopathy: prospective, single blinded, randomised clinical trial. Br J Sports Med. 2018;52(22):1464-1472. doi:10.1136/bjsports-2018-k1662rep
- Patricio Cordeiro TT, Rocha EAB, Scattone Silva R. Effects of exercise-based interventions on gluteal tendinopathy. Systematic review with meta-analysis. Sci Rep. 2024;14(1):3343. Published 2024 Feb 9. doi:10.1038/s41598-024-53283-x
- Clifford C, Paul L, Syme G, Millar NL. Isometric versus isotonic exercise for greater trochanteric pain syndrome: a randomised controlled pilot study. BMJ Open Sport Exerc Med. 2019;5(1):e000558. Published 2019 Sep 21. doi:10.1136/bmjsem-2019-000558
- Mellor R, Kasza J, Grimaldi A, Hodges P, Bennell K, Vicenzino B. Mediators and Moderators of Education Plus Exercise on Perceived Improvement in Individuals With Gluteal Tendinopathy: An Exploratory Analysis of a 3-Arm Randomized Trial. J Orthop Sports Phys Ther. 2022;52(12):826-836. doi:10.2519/jospt.2022.11261
- Plinsinga ML, Coombes BK, Mellor R, et al. Psychological factors not strength deficits are associated with severity of gluteal tendinopathy: A cross-sectional study. Eur J Pain. 2018;22(6):1124-1133. doi:10.1002/ejp.1199
- Grimaldi A, Mellor R, Nasser A, Vicenzino B, Hunter DJ. Current and future advances in practice: tendinopathies of the hip. Rheumatol Adv Pract. 2024;8(2):rkae022. Published 2024 Apr 10. doi:10.1093/rap/rkae022
- Pavlova AV, Shim JSC, Moss R, et al. Effect of resistance exercise dose components for tendinopathy management: a systematic review with meta-analysis. Br J Sports Med. 2023;57(20):1327-1334. doi:10.1136/bjsports-2022-105754
- Rompe JD, Segal NA, Cacchio A, Furia JP, Morral A, Maffulli N. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanter pain syndrome. Am J Sports Med. 2009;37(10):1981-1990. doi:10.1177/0363546509334374
- Dean BJ, Lostis E, Oakley T, Rombach I, Morrey ME, Carr AJ. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014;43(4):570-576. doi:10.1016/j.semarthrit.2013.08.006
- Lange J, Tvedesøe C, Lund B, Bohn MB. Low prevalence of trochanteric bursitis in patients with refractory lateral hip pain. Dan Med J. 2022;69(7):A09210714. Published 2022 Jun 15.
- Robinson NA, Spratford W, Welvaert M, Gaida J, Fearon AM. Does Dynamic Tape change the walking biomechanics of women with greater trochanteric pain syndrome? A blinded randomised controlled crossover trial. Gait Posture. 2019;70:275-283. doi:10.1016/j.gaitpost.2019.02.031
- Fitzpatrick J, Bulsara MK, O’Donnell J, Zheng MH. Leucocyte-Rich Platelet-Rich Plasma Treatment of Gluteus Medius and Minimus Tendinopathy: A Double-Blind Randomized Controlled Trial With 2-Year Follow-up. Am J Sports Med. 2019;47(5):1130-1137. doi:10.1177/0363546519826969
- Migliorini F, Kader N, Eschweiler J, Tingart M, Maffulli N. Platelet-rich plasma versus steroids injections for greater trochanter pain syndrome: a systematic review and meta-analysis. Br Med Bull. 2021;139(1):86-99. doi:10.1093/bmb/ldab018
- Ramon S, Russo S, Santoboni F, et al. Focused Shockwave Treatment for Greater Trochanteric Pain Syndrome: A Multicenter, Randomized, Controlled Clinical Trial. J Bone Joint Surg Am. 2020;102(15):1305-1311. doi:10.2106/JBJS.20.00093
- Seo KH, Lee JY, Yoon K, et al. Long-term outcome of low-energy extracorporeal shockwave therapy on gluteal tendinopathy documented by magnetic resonance imaging. PLoS One. 2018;13(7):e0197460. Published 2018 Jul 17. doi:10.1371/journal.pone.0197460
- Manning, B. How shockwave therapy helps heal sports and overuse injuries [internet]. Dallas: UT Southwestern Medical Center; 2022 (cited: 2024 July 23). Available from: https://utswmed.org/medblog/sports-injuries-shockwave-therapy/
- Heaver C, Pinches M, Kuiper JH, et al. Greater trochanteric pain syndrome: focused shockwave therapy versus an ultrasound guided injection: a randomised control trial. Hip Int. 2023;33(3):490-499. doi:10.1177/11207000211060396
- Harding D, Cameron L, Monga A, Winter S. Is shockwave therapy effective in the management of greater trochanteric pain syndrome? A systematic review and meta-analysis. Musculoskeletal Care. 2024;22(2):e1892. doi:10.1002/msc.1892
- Ferreira-Dos-Santos G, Hurdle MFB, Tran J, Eldrige JS, Clendenen SR, Agur AMR. Ultrasound-Guided Gluteal Fascial Plane Block for the Treatment of Chronic Refractory Greater Trochanteric Pain Syndrome – Technique Description and Anatomical Correlation Study. Pain Med. 2022;23(11):1875-1881. doi:10.1093/pm/pnac071