Meniscus tears, repairs, and rehab options to preserve tissue function.
Key Words: #MSKPlaybook #Meniscus #MeniscusTear #KneeInjury #KneeOA #ACL #SIFK #Osteoarthritis #SEM
Introduction
- Meniscal knee injuries are commonly seen in MSK clinics and can impact athletic patients at all levels of sport or recreational activity. Injuries are seen in both young patients following acute sports injuries and older adults with underlying degenerative joint disease.
- The management of these injuries has evolved alongside the latest developments on tissue function, imaging modalities, regenerative approaches, acknowledging age related changes and rehabilitation goals.
- We discuss how a community MSK focussed pathway can highlight those that need escalation to “acute knee clinics”, further imaging or specialist orthopaedic or sports medicine input for treatment.
The Meniscus – structure and function
The menisci are crescent-shaped fibrocartilaginous structures located on the lateral and medial sides of the knee joint. They have primarily been thought to assist with the following within the knee joint:
- Mechanical stability
- Shock absorption
- Nutrition
- Lubrication
Anatomical variations, injury due to trauma, or age-related wear can impact these key functions and cause changes to the articular cartilage, weight bearing surfaces of the knee and synovial fluid make-up. Typical symptoms following a meniscus tear include pain, stiffness, knee swelling, a loss of range of motion and mechanical instability or locking.
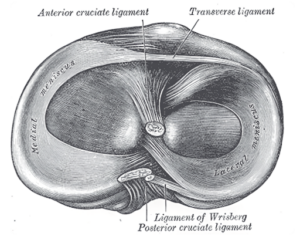
Figure 1: Meniscus anatomy (1)
The menisci are acellular and relatively avascular, with the periphery and capsule supplied by the medial, middle and lateral genicular arteries.
- As the name suggests, the medial genicular artery supplies the medial meniscus, and the lateral genicular artery supplies the lateral meniscus.
- The medial meniscus has a particularly poor blood supply, resulting in poor healing potential following surgery (2).
Histological studies have described age-related changes to the make-up and function of the menisci with increased age and tears associated with; a decreased rate of collagen turnover, increased cytokine and inflammatory signals (3), reduced microvascular blood supply (4), accumulation of glycation end products and reduced mechanical properties (3).
Mechanisms of injury
Acute meniscal tears typically result from a twisting force applied to a weight bearing, flexed knee. In sport, this may be due to contact but can also occur due to sudden changes in direction or when landing from a jump.
On the other hand, degenerative tears often occur without a single clear causative event.
As with other soft tissues, the meniscus is liable to age-related changes that increase the risk of a degenerative tear. Typically, these tears occur due to repetitive stress on a failing tissue, subject to high physical loads and in the context of metabolic risk factors, with little or no history of trauma (5).
Meniscal injuries in the sporting patient: just how common are they?
Acute meniscus tears are primarily observed in younger individuals and athletes, frequently occurring during sport or physical activity. Often, they will present with an associated ACL injury.
- A five-year cohort study found an incidence of 5.1 meniscus tears per 100,000 athlete exposures (training or competitive matches) in high school athletes (6).
- Ligamentous lesion? Lateral likely
- 66.7% of acute ACL injuries have been found to have an associated lateral meniscus tear, while only 4.6% had an associated medial meniscus tear and 6.0% had simultaneous tears of both menisci (7).
- Such concomitant injuries tend to affect the posterior horn, and are often longitudinal ramp lesions (8).
- A key area for injury prevention: knee injuries in the female athlete
-
- In recent years the high incidence of ACL tears in female athletes has been an important point of discussion in sport.
- The risk of ACL injuries in females is 2.8 times higher in football and 3.5 times higher in basketball than males (9).
- Hormonal changes during the menstrual cycle have been proposed as a potential cause (surge in oestrogen during the late follicular phase)(10,11).
- Given the high incidence of meniscus tears associated with ACL injury, it raises questions on whether a similar pattern exists for meniscus tears.
- 68% of meniscus tears in high school athletes were suffered by males, however most of these injuries originated from boys’ American football, a sport in which no female equivalent was considered. In gender-comparable sports (football, basketball, lacrosse, softball), female athletes had more than double the risk of meniscal injury than male counterparts (6).

Figure 2: Risk factors for meniscus tears
The degenerate tear: what do we know about graceful ageing of the meniscal tissue?
Degenerative meniscus tears will typically present in patients aged over 50. Unlike acute tears, the onset of symptoms is chronic, gradually worsening with age. High BMI and repetitive trauma are also known risk factors for degenerative meniscus tears (12). They are also strongly associated with knee osteoarthritis (13).
- Medial Meniscus is more frequently affected
-
- Usually found to be horizontal tears (14).
- Chronic ACL laxity is a risk factor for degenerative meniscus tears
-
- Due to the increased pressure on the meniscus.
- Unlike acute ACL injury, chronic ACL insufficiency often results in a medial meniscus tear (8,15).
- Part of a wider degenerative process in the knee
-
- A 10-year cohort study indicated that individuals with a baseline medial meniscus injury exhibited substantially more advanced osteoarthritis on radiographic assessments compared to those without any meniscus injury.
- The presence of osteophytes on knee X-Rays were associated with increased progression of meniscus tears (16).
- Subchondral insufficiency fractures of the knee (SIFK)
- Strongly associated with degenerative meniscus tears and osteoarthritis (17).
- Increased suspicion of SIFK may arise when a patient can specifically pinpoint a moment when sudden knee pain began, particularly if there is a background of osteoporosis and meniscal symptoms (18,19).
Meniscal tear grading
Several grading criteria exist for meniscal tears, however a clear descriptive report on the size, location and type alongside the following risk factors can help to guide treatment:
- What is the tear type, size and location?
- Is it acute or degenerative?
- Are there any other associated soft tissue of cartilage injuries?
- What is the patient’s age and healing potential of the tear?
- Are there symptoms of instability or a risk of secondary injury or OA in the future?
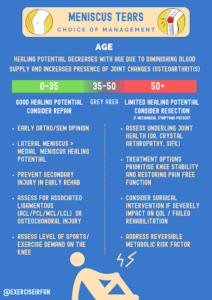
Figure 3: Management options for meniscus tears
| Tear Type | Appearance | Description |
| Peripheral Tear | 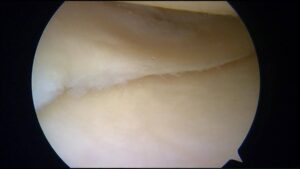 |
Lesion of the outer rim of the meniscus, known as the peripheral zone.
This area has a rich blood supply, so has good healing potential. |
| Horizontal Tear (Cleavage tear) | 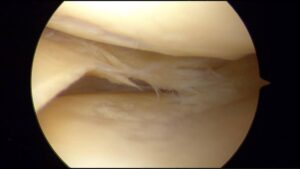 |
Tears are horizontal and parallel to the tibial plateau.
These tears are often degenerative and more likely to be found in older patients. |
| Vertical Tear Repair | 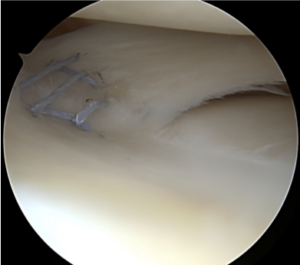 |
Tears extend in a vertical orientation and are more likely to occur acutely in younger patients following trauma.
These are often associated with ACL injuries. |
| Bucket handle tear | 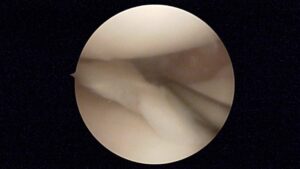 |
Central tear of the meniscus. Peripheral ends are still attached to the knee, thus resembling a ‘bucket-handle’.
The lower image and video show a flipped bucket handle tear – the meniscal tissue has been flipped into the joint between the femoral condyle and the tibial plateau. These are more common in younger patients following an acute tear. |
Table 1: Types of meniscus tears
MSK services should develop close links with orthopaedics to offer fast track referrals to “acute knee” clinics and have the capacity to offer rehab and discuss cases via integrated virtual clinics. An example of a community referral pathway is included below.
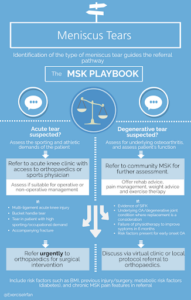
Figure 4: Community referral pathway for meniscus tears
Various clinic-based examinations can be performed to aid the identification of meniscus tears. Findings from these tests should be taken in the context of the background and history of the presenting complaint. The sensitivities and specificities of these are highlighted below:
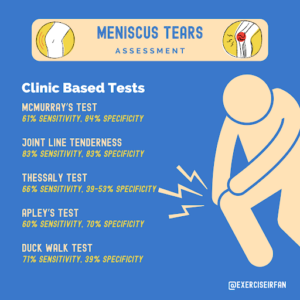
Figure 5: Clinic based tests to aid identification of meniscus tears
Imaging: MSK imaging options to consider for meniscal knee injuries
X-ray/Weightbearing films
Can be used to assess for underlying osteoarthritis. (Kellgren-Lawrence criteria). The addition of Rosenberg views can improve sensitivity.
MRI (See below for MRI examples)
MRI 2-slice-touch rule (using knee arthroscopy to verify diagnosis):
Medial meniscus tear:
- 92% sensitivity
- 90% specificity
Lateral meniscus tear:
- 80% sensitivity
- 95% specificity (20)
Ultrasound
- Initial screening test to assess for associated soft tissue injuries (MCL, LCL, parameniscal cyst, effusion).
- Cannot accurately detect intracapsular injuries/vertical tears of the inner zone of the meniscus.
- Sensitivity of 97% and specificity of 86% for detecting meniscal cysts (21).
MRI Examples
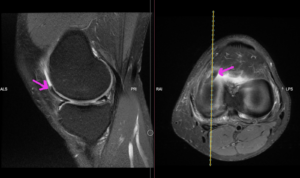
Figure 6: Flipped medial meniscus into the anterior compartment
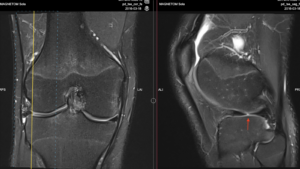
Figure 7: Radial tear of the inner margin of the lateral meniscus
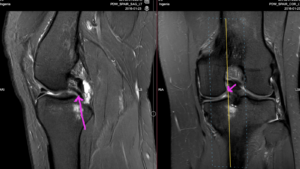
Figure 8: Double PCL. Flap tear of the posterior third of the medial meniscus that has flipped into the intercondylar notch
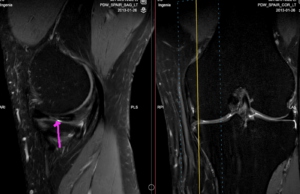
Figure 9: Oblique tear through the middle third of the medial meniscus
Deciding on operative vs non operative management
- Patients are commonly referred into MSK/orthopaedic clinics to discuss treatment options as part of a shared decision-making process.
- A personalised approach should be used to counsel patients, with a focus on pain and function.
- Any meniscus tears should be put in the context of underlying osteoarthritis and the health of the underlying joint.
| Non-operative options | Operative options |
|
|
Table 3: Operative and non-operative treatment options
Injection Therapy
Injection therapies can play an important role in the management of knee osteoarthritis and degenerative meniscus tears. The most commonly used injections include: corticosteroid (steroid), viscosupplementation (hyaluronic acid based), and platelet rich plasma (orthobiologics/regenerative approaches).
- Corticosteroids: effective in reducing pain and inflammation (synovitis), but total steroid burden and frequency of injections should be carefully discussed. This is to reduce the risk of chondrotoxicity (22) or progression of destructive joint disease (23,24).
- Viscosupplementation: The effectiveness of these injections varies by study (25,26), age of patient/underlying tear (27) and method of injection (landmark vs. ultrasound guided). Ultrasound guided hyaluronic acid injections result in better outcomes when compared to landmark based techniques (28). These are still recommended by the American Medical Society for Sports Medicine for patients with osteoarthritis (28), but not routinely advised by NICE (UK) (25). These injections can be considered, in patients that wish to explore non operative treatments to reduce pain whilst avoiding steroid burden.
- Regenerative treatments (Platelet Rich Plasma (PRP)): These are emerging treatments aimed at providing therapeutic benefit for pain and function. Recent systematic reviews and meta-analyses suggest that platelet rich plasma injections outperform corticosteroids, viscosupplementation, and placebo when it comes to symptomatic knee osteoarthritis (12 months follow up) (30,31). However, it is important to point out that not all PRP is the same. Recent clinical trial evidence suggests high dose PRP injections outperform low dose PRP preparations as shown below:
| Authors | Type of Study | Comparison of injection | PRP Dose | n= | Outcomes |
| Bansal et al. 2021 (32) | Prospective, double-blinded, randomised control trial | PRP compared to hyaluronic acid injections | ~10 billion platelets per injection | 150 | PRP at this dose results in significantly better and more sustained improvement in knee OA symptoms than hyaluronic acid.
Significant potential chondro-protection from PRP. |
| Chu et al. 2022 (33) | Prospective, parallel-group, double-blind, multi-center, sham-controlled randomised clinical trial | Pure platelet-rich plasma (P-PRP) compared to placebo saline
1 injection given each week for 3 weeks for all groups |
~4.1 billion platelets per injection | 610 | P-PRP was significantly better at providing sustained improvement of symptoms at 6, 12, 24 and 60 months. |
| Lewis et al. 2022 (34) | Double-blinded, randomised placebo-controlled trial | 3 groups:
3 injections of PRP, 1 injection given each week for 3 weeks for all groups |
~2 billion platelets per injection | 102 | No significant improvement in symptoms seen at 6 weeks, 12 weeks, 6 months and 12 months. |
| Bennell et al. 2021
(35) |
Randomised, 2-group, placebo-controlled, participant-, injector-, and assessor-blinded clinical trial | PRP compared to placebo saline
1 injection given each week for 3 weeks for all groups |
~1 billion platelets per injection | 288 | No significant improvement in knee pain or tibial cartilage volume loss at 12 month follow up. |
| Patel et al.
2024 (36) |
Prospective, triple-blinded, randomised controlled trial | High dose PRP compared to low dose PRP
1 injection given to each group |
~5.65 billion platelets vs ~2.82 billion platelets per injection | 50 | High dose PRP resulted in significantly better improvements in pain and function compared to low dose PRP. |
| Everts et al. 2023 (37) | Retrospective analysis | Comparison of numerous studies using a wide range of platelet dosing and concentrations | See Figure 6. | – | High dose PRP leads to better outcomes, whilst low dose PRP consistently yields less favourable results. |
Table 4: Summary of studies investigating the effect of various PRP doses on patient outcomes.

Figure 10: Higher platelet dosing results in significantly better outcomes as shown in a retrospective analysis by Everts et al. (37)
- Studies vary by PRP protocol, with emerging evidence that high dose volume PRP (50-60ml preparation) preparation is significantly more effective than low dose PRP (10-20ml preparation) (33,37). Current evidence is based on small studies, with a risk of bias.
- In the UK (NICE) (38), PRP can be offered for early or mid-stage knee osteoarthritis subject to governance procedures.
Dietary Supplements
There has been a recent resurgence in the interest of dietary supplements to reduce pain and symptoms related to degenerative knee (OA). These studies are small and have a risk of bias, but patients frequently ask about these during consultations to discuss holistic management options.
Boswellia Serrata
- Description: Herb extracted from the Indian frankincense tree.
- Active compounds: Boswellic acids inhibits the 5-lipoxygenase enzyme which is thought to have an anti-inflammatory effects.
- Outcome: Boswellia serrata can help reduce pain, decrease stiffness, and improve joint function in those with symptomatic knee osteoarthritis (39-41).
Turmeric
- Description: A spice derived from the curcuma longa plant.
- Active compound: Curcumin has anti-inflammatory effects through several biomolecular pathways including inhibition of the NF-kB pathway as well as inhibiting the COX-2 enzyme.
- Outcome: Systematic reviews and meta-analyses have found that curcumin supplementation was significantly more effective than placebo in the improvements of pain, stiffness, and functional scores (40,42,43).
| Authors | Type of Study | Aims of study | Types of Studies Included | No. Studies Included | Summary of Results |
| Liu et al. 2018 (40) | Systematic review and meta-analysis | To investigate the efficacy and safety of dietary supplements for patients with osteoarthritis | Randomised controlled trials | 69 | Collagen hydrolysate, passion fruit peel extract, Curcuma longa extract, Boswellia serrata extract, curcumin, pycnogenol and L-carnitine demonstrated large and clinically important effects for short term pain reduction.
Supplements had no clinically important effects on pain and function at medium-term and long-term follow-ups. |
| Yu et al. 2020 (41) | Systematic review and meta-analysis | To investigate the effects of Boswellia or its extract compared to placebo or western medicine in patients with osteoarthritis | Randomised controlled trials | 7 | Boswellia and its extract may relieve pain, stiffness and joint function in OA.
Recommended treatment duration is 4 weeks. |
| Feng et al. 2022
(42) |
Systematic review and meta-analysis | To investigate the efficacy and safety of dietary curcuminoids for patients with osteoarthritis | Randomised controlled trials | 15 | Curcuminoids were significantly more effective than placebo in improving pain, stiffness and joint function in OA.
They also did not significantly increase the incidence of adverse events compared to placebo. |
| Wang et al. 2021 (43) | Systematic review and meta-analysis | To investigate the efficacy and safety of all types of turmeric extracts for patients with knee osteoarthritis | Randomised controlled trials | 16 | Turmeric extracts significantly improved knee pain and function compared to placebo.
They had similar effects compared to NSAIDs, however had 12% fewer adverse events. |
Table 5: Summary of systematic reviews and meta-analyses investigating the efficacy and safety of dietary supplements for managing osteoarthritis symptoms
Whilst dietary supplements are not routinely advised for knee OA (44), some studies have shown the supplements such as turmeric demonstrate comparable reductions to knee pain and improvements to physical function (compared to NSAID’s) with less risk of adverse events (cardiovascular, renal and gastrointestinal) (43).
Operative options
Meniscus repair
Whether a patient is suitable for surgical meniscus repair depends largely on the meniscus’ healing potential. The following are factors that should be considered:
- The lateral meniscus has a better blood supply than the medial meniscus, so lateral repairs will heal better post-operatively.
- Simultaneous ACL repair has been associated with improved outcomes when compared to isolated meniscus repair (45).
- Patients aged under 35, should be offered surgical meniscus repair due to the good healing potential of the meniscus in this age group.
- Age over 50 is not an absolute contraindication for meniscus repairs, but repair is very rarely performed due to the quality of the tissues and the increased failure rate of the repair.
- For patients between these age groups there is a ‘grey area’, and it is highly debated as to whether repair provides sufficient benefit.
- A recent systematic review suggests that meniscus repairs in patients over 40 do not have an increased failure rate and provide similar functional benefits compared to those under 40 (46).
- It should be noted that studies used in this review had varying definitions of failure; some used reoperation rates, whilst others used radiological findings or ongoing symptoms.
- As a result, failure rates may have been underreported – for example, some patients may have suffered clinical failures, but were not counted if they did not return to theatre.
- With a mean follow up period of 5.5 years, longer follow up will be required to assess longer-term outcomes for these patients.
Ultimately, the decision over whether to attempt repair in this age group should be made taking into account the patient’s activity demands as well as their risk factors for knee joint degeneration (osteoarthritis, high BMI, diabetes etc.). In any case, patients should be counselled on potential risk of repair failure that may result in long term symptoms or further surgery to perform a meniscectomy.
Meniscectomy and its long-term implications
- Meniscectomy, or removal of meniscal tissue is performed in cases where repair is not technically possible, or is considered likely to fail in the longer term due to age of patient, type of tear, vascular supply of the tissues or other reasons.
- Tears that occur in poorly vascularised zones are often non-repairable due to the poor healing potential.
- In degenerative tear types, meniscectomy may be considered when conservative and medical management has failed to improve symptoms; or if there are mechanical symptoms associated with the tear.
- Meniscectomy may involve removal of a part or the whole meniscus (partial or total meniscectomy).
- It is important to counsel patients on the increased risk of developing knee osteoarthritis post meniscectomy.
- A cohort study following patients up 19-years post-meniscectomy have found that even in the absence of radiographic osteoarthritis, patients reported significant long-term symptoms and functional limitations (47).
Meniscus allograph transplants
Meniscus allograph transplants may be indicated following meniscectomy to reduce pain and functional deficits. The following general indications for a transplant:
- Post meniscectomy pain for over 6 months
- <50 years old with symptoms limiting desired activity level
- BMI <35 kgm-2
- Favourable lower limb alignment and stability of the joint
- Minimal radiological evidence of osteoarthritis (Kellgren-Lawrence grade <2)
As per the current METEOR 2 trial (48), patients are deemed unsuitable for meniscus transplantation if they have:
- Symptomatic uncorrected ligament instability
- Full thickness cartilage loss >1cm on MRI
- Inflammatory arthritis affecting the knee joint
- Coronal limb alignment that requires surgery
- Age under 16
Current evidence suggests that meniscus transplants generally result in improved outcomes (49). There is limited data on meniscus transplants in high-level athletes, however one systematic review found that 77% of athletes and active patients could return to sport, with two-thirds able to perform at their pre-injury level (50). The current Meteor 2 trial aims to compare outcomes of meniscus transplants with conservative options post-meniscectomy (48).
Cartilage Therapy
Autologous chondrocyte therapy is used to treat cartilage defects which may present in conjunction with meniscal tears in young patients. This is a newer treatment that may be offered to patients who have:
- A distinct cartilage lesion >2cm2
- Not had previous cartilage surgery in the knee
- Minimal osteoarthritis
- A stable joint
- Not had previous meniscectomy
The aim of this procedure is to generate hyaline-like cartilage, in order to restore areas of damage. Outcomes are generally good, with significantly improved sports activity and return to pre-injury level of sports after 5 years (51,52). Autologous chondrocyte therapy has been shown to provide long-term durability at 11-year follow-up (53).
Subchondral insufficiency fracture of the knee (SIFK)
- In older adults with osteoarthritis and metabolic risk factors, repetitive overload can lead to SIFK and end stage osteoarthritis needing joint replacement.
- Medial meniscal root tears, and post meniscectomy patients are at risk, due to increased loading of the subchondral bone (17,19).
- In the early stages, conservative treatment with protected weight bearing, weight loss counselling and pain management are advised (19).
- Definitive treatment with orthopaedic intervention (joint replacement) is often required.
Managing tears in the active patient that wants to stay active
- Counselling and explaining how to manage meniscus tears in active patients, is a common query in sports medicine clinics.
- Meniscal tears are increasingly common in patients age >40 and can often be an incidental finding during imaging workups.
- In one study of novice marathon runners, 36% of middle-aged novice runners had evidence of a meniscus tear at baseline (54). The presence of a meniscus tear did not predict run time or the chance of completing the marathon, so a personalised approach to support knee health is usually advocated.
- High BMI, osteophytes at the joint margin (55), and running loads >40 miles/week (56) are all associated with an increased risk of meniscal tear progression and reporting pain in runners. It is important to counsel patients on medical and orthopaedic risk factors, alongside rehab discussions.
- A study of High volume (ultra-marathon) runners showed that knee cartilage can adapt and respond to load (57), and further studies have shown that recreational running does not increase the risk of progression to OA. These challenges the previously held views that exercise can increase the risk of OA progression.
Conclusion
- Patient specific factors such as sporting level, age, healing potential, and mechanism of injury can help guide the treatment and rehabilitation of meniscal tears.
- Knee stability, restoration of function and long-term osteoarthritis risk should guide treatment options.
- Shared decision making should include discussions on operative and non-operative treatment options.
- Newer biological approaches to preserve knee health and function are showing promise in the treatment of meniscal tears and knee osteoarthritis.
Authors and Affiliations: Ryan Linn, Dr Rifat Hassan, Dr Irfan Ahmed, Dr Imran Lasker, Dr Jeffrey Peng, Mr Arman Memarzadeh
Ryan Linn
Final Year Medical Student
University College London (UCL)
Twitter: @Ryan_Linn_
Dr Rifat Hassan
Foundation Year 1 Doctor
Norfolk and Norwich University Hospitals
Twitter: @RifatHassan_
Dr Irfan Ahmed
Honorary Locum Consultant in Musculoskeletal, Sport and Exercise Medicine
Cambridge
Twitter: @ExerciseIrfan
Dr Imran Lasker
Consultant Radiologist with Special Interest in MSK
Mid & South Essex Foundation Trust
Twitter: @DocLasker
Links to MSK/MRI courses: imranlasker.com, radiologyseminars.com. emergencyimaging.co.uk
Dr Jeffrey Peng MD, CAQSM
Sports Medicine Physician
Clinical Assistant Professor (Affiliated); Stanford University School of Medicine, Department of Medicine, Division of Primary Care & Population Health
Twitter: @JeffreyPengMD
YouTube: youtube.com/c/JeffreyPengMD
Website: www.JeffreyPengMD.com
Mr Arman Memarzadeh
MBBS, FRCS (Tr and Orth), PGCME
Consultant in Trauma and Orthopaedics, Knee Surgery Specialist
Cambridge University Hospitals
Twitter: @MyKneeSurgeon
YouTube: https://www.youtube.com/@MyKneeSurgeon
LinkedIn: www.linkedin.com/in/mykneesurgeon
Website: https://mykneesurgeon.com
References
- Gray, H., Lewis, W. H., ed. Anatomy of the Human Body. 20th ed. Philadelphia and New York, Lea & Febiger; 1918
- Farrell C, Shamrock AG, Black AC, et al. Anatomy, Bony Pelvis and Lower Limb: Medial Meniscus. [Updated 2023 Nov 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537276/
- Otani S, Kanamoto T, Oyama S, et al. Meniscus surface texture is associated with degenerative changes in biological and biomechanical properties. Sci Rep. 2022;12:11977.
- Michel PA, Domnick CJ, Raschke MJ, et al. Age-Related Changes in the Microvascular Density of the Human Meniscus. Am J Sports Med. 2021;49(13):3544-3550.
- Howell R, Kumar NS, Patel N, Tom J. Degenerative meniscus: Pathogenesis, diagnosis, and treatment options. World J Orthop. 2014;5(5):597-602.
- Mitchell J, Graham W, Best TM, et al. Epidemiology of meniscal injuries in US high school athletes between 2007 and 2013. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):715-722.
- Nikolić DK. Lateral meniscal tears and their evolution in acute injuries of the anterior cruciate ligament of the knee. Arthroscopic analysis. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):26-30.
- Chahla J, Dean CS, Moatshe G, et al. Meniscal Ramp Lesions: Anatomy, Incidence, Diagnosis, and Treatment. Orthop J Sports Med. 2016;4(7):2325967116657815.
- Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34(2):86-92.
- Martin D, Timmins K, Cowie C, Alty J, Mehta R, Tang A, et al. Injury Incidence Across the Menstrual Cycle in International Footballers. Front Sports Act Living. 2021;3:616999.
- Adachi N, Nawata K, Maeta M, Kurozawa Y. Relationship of the menstrual cycle phase to anterior cruciate ligament injuries in teenaged female athletes. Arch Orthop Trauma Surg. 2008;128(5):473-478.
- Snoeker BA, Bakker EW, Kegel CA, Lucas C. Risk factors for meniscal tears: a systematic review including meta-analysis. J Orthop Sports Phys Ther. 2013;43(6):352-367.
- Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence?. Radiol Clin North Am. 2009;47(4):703-712.
- Shiraev T, Anderson SE, Hope N. Meniscal tear – presentation, diagnosis and management. Aust Fam Physician. 2012;41(4):182-187.
- Cipolla M, Scala A, Gianni E, Puddu G. Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Classification, staging and timing of treatment. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):130-134.
- Ijaz Khan H, Chou L, Aitken D, McBride A, Ding C, Blizzard L, Pelletier JP, Martel-Pelletier J, Cicuttini F, Jones G. Correlation Between Changes in Global Knee Structures Assessed by Magnetic Resonance Imaging and Radiographic Osteoarthritis Changes Over Ten Years in a Midlife Cohort. Arthritis Care Res (Hoboken). 2016;68(7):958-64.
- Hussain ZB, Chahla J, Mandelbaum BR, Gomoll AH, LaPrade RF. The Role of Meniscal Tears in Spontaneous Osteonecrosis of the Knee: A Systematic Review of Suspected Etiology and a Call to Revisit Nomenclature. Am J Sports Med. 2019;47(2):501-507.
- Mears SC, McCarthy EF, Jones LC, Hungerford DS, Mont MA. Characterization and pathological characteristics of spontaneous osteonecrosis of the knee. Iowa Orthop J. 2009;29:38-42.
- Ochi J, Nozaki T, Nimura A, Yamaguchi T, Kitamura N. Subchondral insufficiency fracture of the knee: review of current concepts and radiological differential diagnoses. Jpn J Radiol. 2022;40(5):443-457.
- Wang W, Li Z, Peng HM, et al. Accuracy of MRI Diagnosis of Meniscal Tears of the Knee: A Meta-Analysis and Systematic Review. J Knee Surg. 2021;34(2):121-129.
- Rutten MJ, Collins JM, van Kampen A, Jager GJ. Meniscal cysts: detection with high-resolution sonography. AJR Am J Roentgenol. 1998;171(2):491-6.
- McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017;317(19):1967-1975
- Okike K, King RK, Merchant JC, Toney EA, Lee GY, Yoon HC. Rapidly Destructive Hip Disease Following Intra-Articular Corticosteroid Injection of the Hip. J Bone Joint Surg Am. 2021;103(22):2070-2079
- Wijn SRW, Rovers MM, van Tienen TG, Hannink G. Intra-articular corticosteroid injections increase the risk of requiring knee arthroplasty. Bone Joint J. 2020;102-B(5):586-592.
- Pereira TV, Jüni P, Saadat P, et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ. 2022;378:e069722.
- Peck J, Slovek A, Miro P, et al. A Comprehensive Review of Viscosupplementation in Osteoarthritis of the Knee. Orthop Rev (Pavia). 2021;13(2):25549.
- Pelletier JP, Raynauld JP, Abram F, Dorais M, Delorme P, Martel-Pelletier J. Exploring determinants predicting response to intra-articular hyaluronic acid treatment in symptomatic knee osteoarthritis: 9-year follow-up data from the Osteoarthritis Initiative. Arthritis Res Ther. 2018;20(1):40.
- Lundstrom ZT, Sytsma TT, Greenlund LS. Rethinking Viscosupplementation: Ultrasound- Versus Landmark-Guided Injection for Knee Osteoarthritis. J Ultrasound Med. 2020;39(1):113-117.
- Trojian TH, Concoff AL, Joy SM, et al AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes British Journal of Sports Medicine 2016;50:84-92.
- Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage. 2021;13(1_suppl):364S-375S.
- Qiao X, Yan L, Feng Y, Li X, Zhang K, Lv Z, Xu C, Zhao S, Liu F, Yang X, Tian Z. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Musculoskelet Disord. 2023;24(1):926.
- Bansal H, Leon J, Pont JL, et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy [published correction appears in Sci Rep. 2021 Sep 14;11(1):18612]. Sci Rep. 2021;11(1):3971. Published 2021 Feb 17.
- Chu J, Duan W, Yu Z, et al. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2022;30(12):4063-4071.
- Lewis E, Merghani K, Robertson I, et al. The effectiveness of leucocyte-poor platelet-rich plasma injections on symptomatic early osteoarthritis of the knee: the PEAK randomized controlled trial. Bone Joint J. 2022;104-B(6):663-671.
- Bennell KL, Paterson KL, Metcalf BR, et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA. 2021;326(20):2021-2030.
- Patel S, Gahlaut S, Thami T, Chouhan DK, Jain A, Dhillon MS. Comparison of Conventional Dose Versus Superdose Platelet-Rich Plasma for Knee Osteoarthritis: A Prospective, Triple-Blind, Randomized Clinical Trial. Orthop J Sports Med. 2024;12(2):23259671241227863. Published 2024 Feb 26.
- Everts PA, Lana JF, Onishi K, et al. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines. 2023;11(7):1922.
- National Institute for Health and Care Excellence. Platelet-rich plasma injections for knee osteoarthritis [Internet]. London: NICE; 2019 [cited 2024 February 12]. Available from: https://www.nice.org.uk/guidance/ipg637/chapter/1-Recommendations
- Indian Frankincense [Internet]. Versus Arthritis; c2023 [cited 2024 February 12].
- Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):167-175.
- Yu G, Xiang W, Zhang T, Zeng L, Yang K, Li J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement Med Ther. 2020;20(1):225.
- Feng J, Li Z, Tian L, et al. Efficacy and safety of curcuminoids alone in alleviating pain and dysfunction for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Med Ther. 2022;22(1):276.
- Wang Z, Singh A, Jones G, et al. Efficacy and Safety of Turmeric Extracts for the Treatment of Knee Osteoarthritis: a Systematic Review and Meta-analysis of Randomised Controlled Trials. Curr Rheumatol Rep. 2021;23(2):11.
- What are complementary and alternative treatments? [Internet]. Versus Arthritis; c2023 [cited 2024 February 12].
- Wasserstein D, Dwyer T, Gandhi R, Austin PC, Mahomed N, Ogilvie-Harris D. A matched-cohort population study of reoperation after meniscal repair with and without concomitant anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(2):349-355.
- Jaibaji, R.; Khaleel, F.; Jaibaji, M.; Volpin, A. Outcomes of Meniscal Repair in Patients Aged 40 and Above: A Systematic Review. J. Clin. Med. 2023, 12, 6922.
- Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9(4):316-324.
- Meteor 2 trial protocol. IRAS:307686. University of Warwick, 2022.
- Frank RM, Cole BJ. Meniscus transplantation. Curr Rev Musculoskelet Med. 2015 Dec;8(4):443-50.
- Grassi A, Bailey JR, Filardo G, Samuelsson K, Zaffagnini S, Amendola A. Return to Sport Activity After Meniscal Allograft Transplantation: At What Level and at What Cost? A Systematic Review and Meta-analysis. Sports Health. 2019 Mar/Apr;11(2):123-133.
- Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33-41.
- Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010 Sep 15;92(12):2220-33.
- Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
- Horga LM, Henckel J, Fotiadou A, et al. Can marathon running improve knee damage of middle-aged adults? A prospective cohort study. BMJ Open Sport Exerc Med. 2019;5(1):e000586.
- Khan HI, Aitken D, Ding C, Blizzard L, Pelletier JP, Martel-Pelletier J, Cicuttini F, Jones G. Natural history and clinical significance of meniscal tears over 8 years in a midlife cohort. BMC Musculoskelet Disord. 2016;17:4.
- Fredericson M, Misra AK. Epidemiology and aetiology of marathon running injuries. Sports Med. 2007;37(4-5):437-9.
- Schütz U, Ehrhardt M, Göd S, Billich C, Beer M, Trattnig S. A mobile MRI field study of the biochemical cartilage reaction of the knee joint during a 4,486 km transcontinental multistage ultra-marathon using T2* mapping. Sci Rep. 2020;10(1):8157.
Appendix:
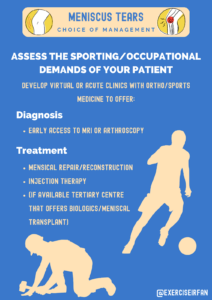
Figure A1: Assessing the sporting/occupational demands of your patient helps to guide management

Figure B1: Risk factors for acute and degenerative meniscus tears