“We can’t see the wood for the trees”
#functionalhypothalamicamenorrhea #contraceptivepill #relativeenergydeficiency
Introduction
The International Olympic Committee (IOC) recently made a new statement about Relative Energy Deficiency in Sport (REDs) (1). Low sexual hormone levels cause hypothalamic amenorrhea, often misunderstood in clinical practice. This blog is an attempt to explain why oral contraceptive pills are not indicated in this context.
Where have the periods gone ?
When periods stops for over 3 months, it is called “secondary amenorrhea”. Functional Hypothalamic Amenorrhea (FHA) is a common cause of secondary amenorrhea and infertility in women, which is a clinical sign of REDs. Approximately 50% of exercising women may experience menstrual disorders, whether they engage in high-performance sports or not (2). Low energy availability disrupts the hypothalamus in FHA (3–5), causing reduced GnRH production, lowered LH pulse, and compromised ovarian function (See Figure 1). The long-term impacts of FHA, including varied metabolic effects, are significant (6,7). So far, the most important area of research is bone mineral density, which may be severely altered during FHA and it is currently unknown if severe alterations of BMD are fully reversible (8).

Figure 1. Main endocrine effects of functional hypothalamic amenorrhea. Estradiol deficiency related to athletic amenorrhea leads to weaker bone mineral density and modified microarchitecture, increasing the risk of low bone mass and bone injury.
Bones and Oral contraceptive Pills are not best friends
Hypoestrogenemia and the lack of energy availability lead to decreased bone mineral density by reducing anabolic hormones (9–12). While menstrual cycle resumption is ideal for bone recovery, many women receive oral contraceptives without knowing they don’t restore natural menstruation. It seems to be postulated that oral oestrogen can replace endogenous production of oestradiol for bone formation, yet research doesn’t support this. Even more alarming, several controlled trials (13,15) indicated a short-term decrease in bone formation markers following a treatment consisting in a combined oral contraceptive in women with FHA. The guidelines established by the Endocrine Society, the International Olympic Committee and The Female Athlete Triad Coalition agree that combined oral contraceptives are not indicated in the context of functional hypothalamic amenorrhea (9,16).
What are the current recommendations regarding bone health?
Recent decades have been pivotal in the management of functional hypothalamic amenorrhea. Well-designed research has revealed that oral estrogen might shutdown IGF-1 and free androgens concentrations, whereas transdermal oestrogen administration (the physiological form of estrogen) seems to lead to better results regarding BMD in women with anorexia nervosa and FHA (17,18). Recently, the British Journal of Medicine published a systematic review and meta-analysis on the effect of oral and transdermal oestrogen therapy on BMD in FHA and confirmed these findings (19).
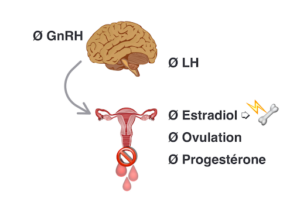
Figure 2. Oral contraceptive pill in the context of Fonctional Hypothalamic Amenorrhea downregulates the production of GnRH which turns off LH, natural production of 17 β-estradiol and ovulation.The most commun form found in oral contraceptive pill ethinyl estradiol also shuts down IGF-1 in the liver, an anabolic hormone which contributes to building bone tissue. Bone mineral density is not preserved and bone microarchitecture is also altered. *Intentionally, we do not mention any specific type of OCP as all of them would have the same effect on GnRH downregulation and bone health
Currently, the recovery of natural menstrual cycles is the optimal approach for improving low BMD in individuals with FHA. However, in some cases, transdermal estradiol combined with a progestin may be helpful. Particularly, the Endocrine Society has given clear advice on this subject: it suggests “against patients with FHA using contraceptive pills for the sole purpose of regaining menses or improving BMD” (16).
Conclusion
- FHA is a common cause of infertility in women and can have severe long-term health consequences, not limited to reproductive or bone health (6).
- The suppression of sex hormones can not be considered as a “natural contraception” and cannot be normalized if a pregnancy is unwanted.
- More than three decades after initial research, it is imperative for all professionals to acknowledge that prescribing oral oestrogen to women with FHA merely masks the underlying cause without promoting healing.
- The utilization of contraceptive pills is a way to silent reproductive function, not a menstrual regulation. In the context of FHA, this approach can be likened to applying a superficial remedy to a single tree, while neglecting the extensive forest of metabolic, endocrine and occasionally psychological complexities associated with the conditions.
Declarations of interest : none.
Authors affiliations :
- Barbara Vulpinari-Grajon, MSc Nutrition Middlesex University
- Manon Dauvergne, Physiotherapist, Msc Sport Science, PhD candidate, Université de La Réunion, Le Tampon, France
- Dr Nicky Keay, Honorary Clinical Lecturer UCL, Author of “Hormones, Health and Human Performance”
Bibliography
- Mountjoy M, Ackerman KE, Bailey DM, Burke LM, Constantini N, Hackney AC, et al. 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br J Sports Med. 1 sept 2023;57(17):1073-97.
- De Souza MJ, Toombs RJ, Scheid JL, O’Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Human Reproduction. 1 févr 2010;25(2):491-503.
- Loucks AB, Thuma JR. Luteinizing Hormone Pulsatility Is Disrupted at a Threshold of Energy Availability in Regularly Menstruating Women. The Journal of Clinical Endocrinology & Metabolism. 1 janv 2003;88(1):297-311.
- Williams NI, Leidy HJ, Hill BR, Lieberman JL, Legro RS, Souza MJD. Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. American Journal of Physiology-Endocrinology and Metabolism. janv 2015;308(1):E29-39.
- Lieberman JL, De Souza MJ, Wagstaff DA, Williams NI. Menstrual Disruption with Exercise Is Not Linked to an Energy Availability Threshold. Medicine & Science in Sports & Exercise. mars 2018;50(3):551.
- Shufelt CL, Torbati T, Dutra E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin Reprod Med. mai 2017;35(03):256-62.
- Elliott-Sale KJ, Tenforde AS, Parziale AL, Holtzman B, Ackerman KE. Endocrine Effects of Relative Energy Deficiency in Sport. International Journal of Sport Nutrition and Exercise Metabolism. 1 juill 2018;28(4):335-49.
- De Souza MJ, Ricker EA, Mallinson RJ, Allaway HC, Koltun KJ, Strock NC, et al. Bone mineral density in response to increased energy intake in exercising women with oligomenorrhea/amenorrhea: the REFUEL randomized controlled trial. The American Journal of Clinical Nutrition. 1 juin 2022;115(6):1457-72.
- Souza MJD, Nattiv A, Joy E, Misra M, Williams NI, Mallinson RJ, et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 1 févr 2014;48(4):289-289.
- Mountjoy M, Sundgot-Borgen JK, Burke LM, Ackerman KE, Blauwet C, Constantini N, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 1 juin 2018;52(11):687-97.
- Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. oct 2012;51(4):680-7.
- Southmayd EA, Williams NI, Mallinson RJ, De Souza MJ. Energy Deficiency Suppresses Bone Turnover in Exercising Women With Menstrual Disturbances. The Journal of Clinical Endocrinology & Metabolism. 1 août 2019;104(8):3131-45.
- Vescovi JD, Jamal SA, De Souza MJ. Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: a systematic review of the literature. Osteoporos Int. 1 avr 2008;19(4):465-78.
- Altayar O, Al Nofal A, Carranza Leon BG, Prokop LJ, Wang Z, Murad MH. Treatments to Prevent Bone Loss in Functional Hypothalamic Amenorrhea: A Systematic Review and Meta-Analysis. Journal of the Endocrine Society. 1 mai 2017;1(5):500-11.
- Grinspoon SK, Friedman AJ, Miller KK, Lippman J, Olson WH, Warren MP. Effects of a Triphasic Combination Oral Contraceptive Containing Norgestimate/Ethinyl Estradiol on Biochemical Markers of Bone Metabolism in Young Women with Osteopenia Secondary to Hypothalamic Amenorrhea. The Journal of Clinical Endocrinology & Metabolism. 1 août 2003;88(8):3651-6.
- Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 1 mai 2017;102(5):1413-39.
- Ackerman KE, Singhal V, Baskaran C, Slattery M, Reyes KJC, Toth A, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 1 févr 2019;53(4):229-36.
- Nose-Ogura S, Yoshino O, Kanatani M, Dohi M, Tabei K, Harada M, et al. Effect of transdermal estradiol therapy on bone mineral density of amenorrheic female athletes. Scandinavian Journal of Medicine & Science in Sports. 2020;30(8):1379-86.
- Aalberg K, Stavem K, Norheim F, Russell MB, Chaibi A. Effect of oral and transdermal oestrogen therapy on bone mineral density in functional hypothalamic amenorrhoea: a systematic review and meta-analysis. BMJ Open Sport & Exercise Medicine. 1 juill 2021;7(3):e001112.