Metronome-paced rehabilitation for tendinopathy
Tendon neuroplastic training (TNT) as a novel tendinopathy rehabilitation protocol is based on the premise that neural changes may precipitate the characteristic chronic, i.e. perpetual pain experienced by patients with tendinopathy. TNT considers motor control changes to be an important factor in aberrant tendon load.
Tendon Neuroplastic Training
The concept of TNT was first described in treating patients with patellar tendinopathy by Rio and colleagues 1.
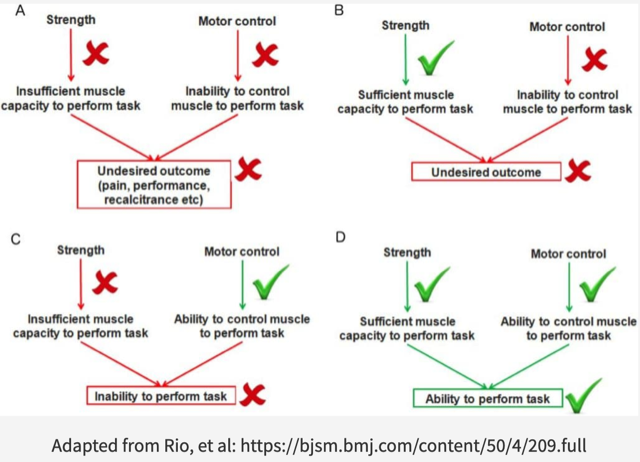
Persistent or chronic pain is a major hallmark of tendinopathy. Chronic pain is associated with corticospinal and neuromuscular adaptations. Altered motor control, or motor performance, most likely a protective strategy to decrease pain or increase performance, could theoretically negatively influence tendon load and perpetuate nociceptive input. As such, alterations to the corticospinal control of the muscle must be taken into consideration when designing appropriate tendinopathy rehabilitation programs. TNT combines knowledge from traditional tendon rehabilitation loading protocols and the neuroplastic effects following both strength training and the pathogenesis of tendinopathy on e.g. the primary motor cortex, corticospinal excitability and motor control, i.e. motor unit recruitment and activation.
TNT addresses the potential benefits of restoring ‘corticospinal control of the muscle-tendon complex(s)’ or muscle drive, which is currently missing in typical tendon rehabilitation protocols. Current clinical rehabilitative approaches primarily focus on building strength of the local damaged tissue (improvement of muscle architecture) and promote tendon mechanical properties such as the matrix. These programs are usually self-paced resistance trainings consisting of eccentric exercises, isometric exercises, a combination or traditional heavy slow resistance training with guidance of repetitions, sets and load (graded activity). However, due to the self-paced nature of the exercises, these programs do not address muscle drive, i.e. do not induce changes to the motor cortex 2,3 which may be another important factor to consider.
TNT appreciates important and ambiguous findings relating to the intricate changes in motor control due to tendinopathy.
Researchers observed bilateral signs of injury and inflammation, i.e. biological changes associated with the pathogenesis of tendinopathy, in the exercised as well as non-exercised tendons of rat forelimbs after subjecting rats to chronic repetitive reaching (voluntary forelimb repetitive reaching and grasping tasks) 4.
The observation that unilaterally exercise-induced tendinopathy evokes bilateral tendon pathology indicates the involvement of centrally mediated processes.
More animal research demonstrates the presence of local cellular tissue responses and signs of injury and inflammation in the uninvolved/unaffected limb 5 which indicate involvement of central neuronal mechanisms that, by extension, may modulate motor control. What about human patients with tendinopathy? Do we find evidence for motor control changes in patients with tendinopathy?
Indeed, a 2014 review and meta-analysis suggests involvement of the central nervous system in human patients with tendon pain, specifically in patients with unilateral tendinopathy of the proximal lateral elbow, and discusses bilateral deficits in sensory and motor systems 6. These motor control changes – increased cortical inhibition and increased cortical spinal excitability – reflect the difference in balance between inhibition and excitation of motor control and are associated with phasic, i.e. intermittent pain, which nicely describes chronic tendon pain in most patients. Though most likely a protective strategy to decrease pain and/or increase performance, enduring motor control changes, i.e. non resolution of this protective strategy may pose a risk factor for recurrence of symptoms which is prevalent in patients with tendinopathies 1,7–9.
The concept of TNT explored ‘external pacing’ (EP) in two RT loading protocols for patients with patellofemoral tendinopathy (PT). EP was the facilitated through cues via metronome and/or verbal instructions. The authors concluded that muscle drive, i.e. muscle recruitment patterns and thus tendon load, was positively influenced which may also have contributed to, albeit short term, pain reduction in patients with PT. Research also demonstrated the positive effects of TNT in two patients with lateral elbow tendinopathy 10.
TNT incorporates interesting new knowledge relating to the relationship between tendinopathy-associated persisting pain and concomitant changes in motor control. Retraining the brain to decrease pain with EP is easy to implement and may prove to become a valuable addition to traditional tendon rehabilitation loading protocols 10.
Key takeaways
- TNT is a novel tendinopathy treatment protocol which addresses the involvement of the central nervous system for ongoing debilitating tendinopathy symptoms
- External-paced isoweight training (with e.g. an metronome) positively effects muscle drive, tendon load and tendon pain
- Future tendinopathy interventions should target both tensile capacity and motor control deficits
Author and Affiliations:
By Michiel R.M. Twiss, @physiotwiss
Michiel R.M. Twiss is a Dutch physiotherapist in Buchs SG, Switzerland. He has a keen interest in systematic reviews and meta-analyses in gerontology research and specifically strength and conditioning training in old age. He holds a private practice in Buchs, Sankt Gallen, Switzerland.
References:
- Rio, E. et al. Tendon neuroplastic training: changing the way we think about tendon rehabilitation: a narrative review. Br. J. Sports Med. 50, 209–215 (2016).
- Bayona, N. A., Bitensky, J., Salter, K. & Teasell, R. The role of task-specific training in rehabilitation therapies. Top. Stroke Rehabil. 12, 58–65 (2005).
- Leung, M., Rantalainen, T., Teo, W.-P. & Kidgell, D. Motor cortex excitability is not differentially modulated following skill and strength training. Neuroscience 305, 99–108 (2015).
- Barbe, M. F. et al. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 21, 167–176 (2003).
- Andersson, G. et al. Tenocyte hypercellularity and vascular proliferation in a rabbit model of tendinopathy: contralateral effects suggest the involvement of central neuronal mechanisms. Br. J. Sports Med. 45, 399–406 (2011).
- Heales, L. J., Lim, E. C. W., Hodges, P. W. & Vicenzino, B. Sensory and motor deficits exist on the non-injured side of patients with unilateral tendon pain and disability—implications for central nervous system involvement: a systematic review with meta-analysis. Br. J. Sports Med. 48, 1400–1406 (2014).
- Abat, F. et al. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part I: biology, biomechanics, anatomy and an exercise-based approach. J. Exp. Orthop. 4, (2017).
- Schwartz, A., Watson, J. N. & Hutchinson, M. R. Patellar Tendinopathy. Sports Health 7, 415–420 (2015).
- Paavola, M., Kannus, P., Paakkala, T., Pasanen, M. & Järvinen, M. Long-term prognosis of patients with achilles tendinopathy. An observational 8-year follow-up study. Am. J. Sports Med. 28, 634–642 (2000).
- Welsh, P. Tendon neuroplastic training for lateral elbow tendinopathy: 2 case reports. J. Can. Chiropr. Assoc. 62, 98–104 (2018).