The retraction yesterday of two publications, one in The Lancet and one in the New England Journal of Medicine, reflects a current major problem with research into the management of covid-19—that we have abandoned the tenets of evidence based medicine.
In my recent litany on the subject I mentioned the following problems:
- attempts to prove weak hypotheses …
- … in poorly designed clinical trials;
- failure to test strong hypotheses;
- premature reporting of results in press releases and in non-peer reviewed publications;
- premature termination of trials before clear outcomes have occurred;
- changing prespecified endpoints without explanation and reporting other endpoints instead;
- rapid, and perhaps premature, authorization of new medicines.
To all of these I might have added the rush to publish in peer reviewed journals and publication accompanied by striking press releases. Here’s an example from my own experience. A few weeks ago a learned journal asked me to review a paper. Two days later, on a Saturday afternoon, the editor sent me an email: “We recently invited you to review the above-referenced manuscript. In the meantime, we have received a sufficient number of reviews to complete the review process, and unfortunately, we must proceed without your valued input in order to ensure a fast manuscript review process.” The authors had explained why the medicine they were discussing might be effective, despite the fact that they had also asserted, from mechanistic reasoning, that it should not. I had thought to suggest that they might change their emphasis, and instead discuss why the drug should be used at all, given that they believed that it was unlikely to be effective. I turned my attention to other matters.
The word retraction comes from the Latin verb trahere (past participle tractus), to drag, pull, or draw or carry along in several senses, and by extension to convey an idea mentally. The addition of the usual suffixes gives us a swathe of English words. The abstract of a paper may attract you to read it, or at least an extract, rather than the more protracted version; it may prompt a train of thought that will distract you for a while, but when it is retracted, you may realize that it is not the treat you thought it was, and the event may detract from your propensity to believe anything published thereafter in the journal that published it.
In a review of retracted publications, the highest proportions came from Egypt (304 retractions per million publications), Iran (295), South Korea (219), China (216), India (193), Singapore (161), Thailand (157), Japan (132), Turkey (124), the Netherlands (119), and Germany (108). The authors described twelve reasons for retraction in 2088 cases: plagiarism (19.2%); data fabrication or falsification (17.7%); suspected fraud (17.5%); scientific errors (14.4%); duplicate publication (6.3%); authors’ disputes (3.2%); violation of ethics (2.7%); publishers’ errors (2.6%); problems with copyright (1.1%); manipulation of peer review (0.4%); problems with funding (0.1%); combinations (6.2%); unknown (8.5%).
The story of the recent retractions is salutary. The Lancet issued a press release announcing the forthcoming publication, on 22 May, of a retrospective analysis of data from over 96 000 patients in 671 hospitals worldwide. Mortality was reportedly increased in those who had received a 4-aminoquinolone (chloroquine or hydroxychloroquine), and more so in those who had also received a macrolide antibiotic (azithromycin or clarithromycin). The results were believable, since both types of drug can prolong the electrocardiographic QT interval, with a risk of the polymorphous ventricular tachycardia called torsades de pointes (Figure 1), and the combinations are better avoided. However, it was not long before doubts began to be raised about the provenance and accuracy of the data, and the authors have since announced that since they cannot vouch for the data they have retracted the paper.
Despite this retraction, the reported effects may be real; we can’t tell. However, two UK trials, COPCOV and PRINCIPLE, were immediately halted before the retraction, and now today the RECOVERY investigators have announced that they too will no longer recruit participants into the hydroxychloroquine arm of their trial, following an analysis of the latest data. They randomized 1542 patients to hydroxychloroquine and compared them with 3132 patients randomized to usual care alone. There was no significant difference in the primary endpoint of 28-day mortality (25.7% hydroxychloroquine vs. 23.5% usual care; hazard ratio 1.11 [95% confidence interval 0.98-1.26]; P=0.10) and no evidence of beneficial effects on hospital stay duration or other outcomes. They concluded that “These data convincingly rule out any meaningful mortality benefit of hydroxychloroquine in patients hospitalised with COVID-19.” Indeed the data suggest that it is likely that there is increased mortality in those given hydroxychloroquine, the lower 95% confidence limit being only just below 1. The WHO have also halted the hydroxychloroquine arm of their SOLIDARITY trial.
This must surely be the end of the road for the 4-aminoquinolines, chloroquine and hydroxychloroquine, in the treatment of covid-19.
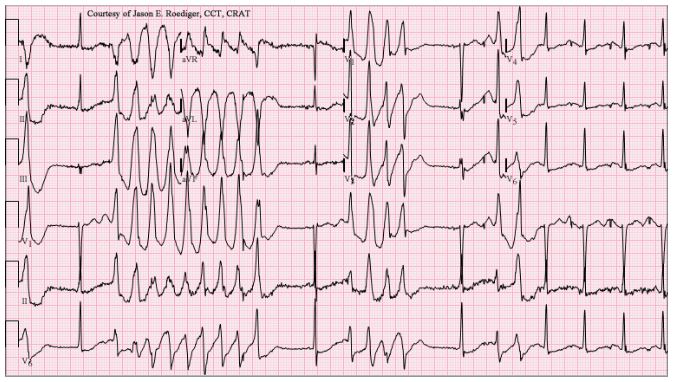
Figure 1. An example of torsades de pointes (source: Wikimedia Commons)
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.