DNA damage is recognised as the first step in the development of cancer, but also many other chronical diseases as well as the aging process. Oxidative stress and inflammation are closely intertwined in disease pathogenesis [1]. Oxidative stress is characterized by an imbalance between formation of reactive oxygen species (ROS) and antioxidant defense mechanisms, induced by endogenous and exogeneous factors [2]. Most chemotherapeutics generate ROS in cancer cells which causes DNA damage and trigger cell death. Unfortunately, they also damage healthy cells [3]. Increasing evidence indicate that dietary factors and exercise affect DNA stability by upregulating antioxidant defense system and modulating repair capacity (Figure 1).
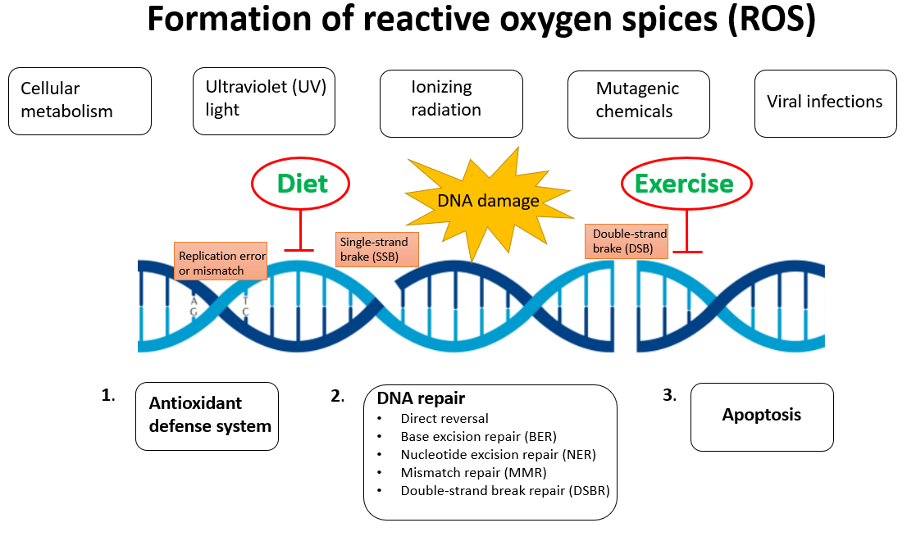
Why should diet and exercise be used as a part of a cancer treatment regimen?
In recent decades, progress in early detection and treatment of cancer has led to an increase in cancer survivors, defined as all people who have been diagnosed with cancer, including before, during and after treatment. Treatment regimes in cancer patients are intended to stop cancer progression but also prolong patients’ survival and reduce the risk for relapse [5]. In addition, there is an intention to reduce the negative side effects of cancer therapy which many cancer patients are struggling with years after treatment.
A large number of bioactive compounds has been identified in plant food (e.g. whole grains, berries, spices, nuts, coffee, specific fruit and vegetables) with the potential of dampening oxidative stress and inflammation [6-10]. These compounds provides a protection against DNA damage via different modes of action, including increased resistance to oxidative damage and induction of alterations of DNA-repair processes [11]. In numerous clinical trials, antioxidant-rich foods and beverages have resulted in reduced DNA damage, a higher antioxidant capacity and increased DNA repair capacity [12-16]. This indicate that a prudent diet rich in specific plant-foods may be beneficial for cancer survivors, especially individuals with molecular signatures creating major oxidative stress and inflammation.
Acute exercise contributes to a transient increased production of ROS due to an increased activity in the mitochondria and other metabolic pathways, which in turn may lead to oxidative stress and DNA damage [17]. The increase in ROS will depend on the characteristics of the exercise, such as intensity, time, and frequency [18]. However, it seems that DNA repair likely occurs within a least 3 days [17]. Importantly, regular exercise has been shown to up-regulate the antioxidant defense system and enhance DNA repair processes [19]. On the other hand, very high levels of activity may led to DNA damage, and it has therefore been proposed that the relationship between exercise, ROS and DNA damage may follow a U-shaped curve [19]:
- Inactivity results in higher risk of DNA damage
- Moderate/regular exercise may protect against DNA damage
- High exercise/overtraining may increase DNA damage
However, it is unclear where the threshold limit exists between the beneficial effects of regular exercise level and the point of overtraining associated with higher oxidative damage and insufficient DNA repair. Considering this, regular exercise and aerobic exercise in particular may contribute to reduced DNA damage due to a higher antioxidant capacity and increased DNA repair capacity.
Disease outcomes and survival
Dietary factor’s and exercise’ ability to modulate DNA stability adds a new dimension to the mechanisms linking diet, physical activity and cancer. There is strong evidence for the role that diet and physical activity play in cancer prevention, but the relations to disease outcome and survival is less studied [20].
Historically, dietary advice to cancer survivors focused on maintaining a persons’ energy intake and micronutrient sufficiency as well as mitigating the effects of unintentional weight change, loss of muscle mass, nausea, and gastrointestinal toxicity. The question now is whether we also should focus on foods and drinks that have been suggested to dampen oxidative stress and inflammation, which in turn may protect against DNA damage. Antioxidant-rich foods and drinks can boost the antioxidant defense system, moreover, there are indications that it will enhance DNA repair capacity. Both mechanisms can theoretically increase treatment tolerance, reduce risk of recurrence, and reduce long-term side effects in cancer patients.
Physical activity has emerged as an important factor to improve cancer outcomes. There is strong evidence that physical activity confers diverse benefits related to fatigue, depression, quality in life, physical function, body composition, and cardiorespiratory fitness [21]. In addition, physiological and biological process during and after exercise may also impact the rate of tumor growth and boost the immune system, which in turn may affect therapeutic toxicity and tolerance [22]. In recent years, high intensity interval training (HIIT) has received greater focus in improving physical fitness and health-related outcomes in cancer survivors [23]. In this context, benefit of total physical activity for DNA repair appears to be driven primarily by high intensity exercise [24]. Given that DNA repair counteracts DNA damage, and excessive DNA damage is involved in carcinogenesis, high intensity exercise may be beneficial in order to reduce risk of relapse as well as other chronical diseases in cancer survivors.
Conclusion
Cancer is associated with a DNA instability due to increased damage to DNA. DNA repair capacity is regarded as an indicator of individual cancer susceptibility and growing evidence suggests that DNA repair capacity is an important factor in tolerance to chemotherapeutic agents [25]. Dietary factors and exercise can protect against DNA damage by upregulating antioxidant defense system and modulating repair capacity. However, there is a need for prospective studies that monitor these markers related to diet and exercise which will be of great importance for cancer survivors.
Author and affiliations:
Anne Lene Nordengen, BSc in Sports Biology and MSc in Sports Physiology from The Norwegian School of Sport Sciences. She is currently PhD student at the Department of Sport Science and Physical Education, University of Agder, and is also affiliated with the Department of Nutrition, University of Oslo, and NorGenoTech AS (Oslo Cancer Cluster), Norway.
References:
1. Hanahan, D. and R.A. Weinberg, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646-74.
2. Thanan, R., et al., Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci, 2014. 16(1): p. 193-217.
3. Srinivas, U.S., et al., ROS and the DNA damage response in cancer. Redox Biol, 2019. 25: p. 101084.
4. Rowe, L.A., N. Degtyareva, and P.W. Doetsch, DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic Biol Med, 2008. 45(8): p. 1167-77.
5. Vodicka, P., et al., DNA damage and repair measured by comet assay in cancer patients. Mutat Res Genet Toxicol Environ Mutagen, 2019. 843: p. 95-110.
6. Jacobs, D.R., Jr., L.F. Andersen, and R. Blomhoff, Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am J Clin Nutr, 2007. 85(6): p. 1606-14.
7. Karlsen, A., et al., Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr, 2010. 49(6): p. 345-55.
8. Kolberg, M., et al., Tomato paste alters NF-kappaB and cancer-related mRNA expression in prostate cancer cells, xenografts, and xenograft microenvironment. Nutr Cancer, 2015. 67(2): p. 305-15.
9. Kolberg, M., et al., Plant extracts of spices and coffee synergistically dampen nuclear factor-kappaB in U937 cells. Nutr Res, 2013. 33(10): p. 817-30.
10. Paur, I., L.M. Austenaa, and R. Blomhoff, Extracts of dietary plants are efficient modulators of nuclear factor kappa B. Food Chem Toxicol, 2008. 46(4): p. 1288-97.
11. Nersesyan, A., et al., Chapter 12. Use of Single-cell Gel Electrophoresis Assays in Dietary Intervention Trials, in The Comet Assay in Toxicology. 2016. p. 314-353.
12. Brevik, A., et al., Supplementation of a western diet with golden kiwifruits (Actinidia chinensis var.’Hort 16A’:) effects on biomarkers of oxidation damage and antioxidant protection. Nutrition Journal, 2011. 10: p. 54.
13. Schipp, D., et al., Consumption of a dark roast coffee blend reduces DNA damage in humans: results from a 4-week randomised controlled study. Eur J Nutr, 2019. 58(8): p. 3199-3206.
14. Gill, C.I., et al., Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am J Clin Nutr, 2007. 85(2): p. 504-10.
15. Moser, B., et al., Impact of spinach consumption on DNA stability in peripheral lymphocytes and on biochemical blood parameters: results of a human intervention trial. Eur J Nutr, 2011. 50(7): p. 587-94.
16. Riso, P., et al., DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis, 2010. 25(6): p. 595-602.
17. Tryfidou, D.V., et al., DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med, 2020. 50(1): p. 103-127.
18. Fisher-Wellman, K. and R.J. Bloomer, Acute exercise and oxidative stress: a 30 year history. Dyn Med, 2009. 8: p. 1.
19. Radak, Z., et al., Exercise, oxidative stress and hormesis. Ageing Res Rev, 2008. 7(1): p. 34-42.
20. Wiseman, M.J., Nutrition and cancer: prevention and survival. Br J Nutr, 2019. 122(5): p. 481-487.
21. Spence, R.R., et al., Physical Activity and Exercise Guidelines for People With Cancer: Why Are They Needed, Who Should Use Them, and When? Semin Oncol Nurs, 2020. 36(5): p. 151075.
22. Hojman, P., et al., Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab, 2018. 27(1): p. 10-
23. Mugele, H., et al., High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv, 2019. 13(2): p. 205-223.
24. Cash, S.W., et al., Recent physical activity in relation to DNA damage and repair using the comet assay. J Phys Act Health, 2014. 11(4): p. 770-6.
25. Kiwerska, K. and K. Szyfter, DNA repair in cancer initiation, progression, and therapy-a double-edged sword. J Appl Genet, 2019. 60(3-4): p. 329-334.