By Rohan Henry
In a 1958 editorial, the first case of growth hormone used as treatment for a medical condition was reported. Since that time, the administered product has changed from being pituitary derived specifically, cadaveric in origin, to recombinant human growth hormone in the United States which occurred in 1985. With this practice shift, the indications surrounding prescribing growth hormone have changed based on its increased supply relative to demand. Additionally, growth hormone use has expanded beyond the initial indication which was to treat a growth hormone deficient state. As such, clinicians should be cognizant of the potential ethical implications which accompany the historical changes that have influenced the prescribing of growth hormone in pediatrics.
Growth Hormone use: Prior to 1985
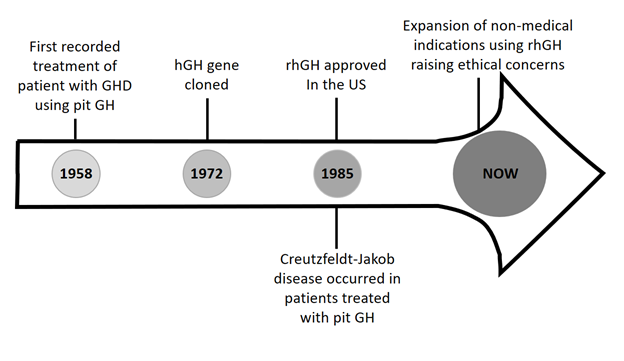
The first reported use of growth hormone (GH) as treatment for growth hormone deficiency (GHD) occurred in 1958 when in the Journal of Clinical Endocrinology and Metabolism, Raben described the height gains of a 17-year-old male with GHD treated with human pituitary derived GH.
With cadaveric GH use, 1 mg human growth hormone (hGH) daily was needed per patient, hence, 365 pituitaries were required annually per patient receiving GH therapy. In the United States (US) the total pool of about 10,000 pituitaries per year allotted for Pediatrics, meant that only 30 patients could be treated under the indication of GHD. Based on the product’s finiteness and its relative scarcity during the 1960’s, research was initiated to advance the existing fund of knowledge.
In the 1960’s under the direction of Dr. Robert Blizzard from the University of Virginia, the National Pituitary Agency was established to organize research and to collect the pituitary extract. This preceded the National Collaborative Growth Hormone treatment project which successfully used hGH to manage 100 children over 10 years(2). To address the limited GH supply in the US relative to its demand, the imposed 5 feet height cap ensured that only the shortest individuals were treated with GH which at that time was solely prescribed under the indication of GHD.
Prior to 1969 there was commercial interest in hGH marketing with a California based company, Calbiochem. This preceded a seminal event in 1972, the elucidation of GH’s biochemical structure which served as a catalyst in the production of recombinant human growth hormone (rhGH) by DNA technology.
Growth Hormone use: After 1985
As with many historical events, the evolution of the GH product into that marketed today occurred by serendipity. Given that 3 adults in the US and 1 in Canada treated with the pituitary derived extract developed Creutzfeldt- Jacob Disease, product marketing abruptly ceased in 1985. Fortuitously, GH produced by recombinant DNA technology was approved by the Food and Drug Administration that same year, resulting in an increased product supply.
This catapulted a practice change, abrogating the need for the original height cap. Today based on abundant GH supply and intense pharmaceutical marketing, product sales have created a multi-billion-dollar industry.
With the increased supply of rhGH it became feasible to study the impact of GH replacement therapy for adults with GHD secondary to diseases involving the pituitary or hypothalamus. Furthermore, though it was speculated that GH therapy may abrogate certain metabolic effects in adulthood which existed in deficient individuals, it could not be proven due to the limited GH supply prior to 1985. As such, even though patients underwent treatment with GH due to childhood onset GHD, administration of rhGH was discontinued after growth plate closure prior to 1987. However, in 1987 the FDA approved GH replacement for adults with diseases of the pituitary gland or hypothalamus classified as having newly diagnosed or acquired GHD based on the recognition of a distinct GH deficiency syndrome in this population. This includes decreased bone mineral density, metabolic abnormalities and lastly, reduced psychosocial well-being and quality of life.
Undoubtedly, the prescribing of GH by pediatric endocrinologists has been influenced by secular trends. Despite an increased sensitivity of the GH assays generated by laboratory technique advancements, there has not been concomitant downward adjustment of cutoff levels below which the diagnosis of GHD is made. In the US and Poland a level of 10 ng/mL is used, whereas a level of 7 ng/mL is utilized in most other countries. Growth hormone sufficiency is proven mainly by doing provocation testing after an agent is administered to elicit its release. Over the years the peak stimulated value below which the diagnosis of GHD is made has changed from 5 mcg/L to 7 mcg/L in 1965 and 1975 respectively and then to 10 mcg/L in 1985, despite the lack of evidence for validating this change and laboratory assay advancements. The reason cited for the change to 10 mcg/L is that is slightly below the mean cutoff level elicited by many agents which are utilized in evaluation of endogenous GH production in a population of children with normal growth.
With the prescribing of GH for GHD not being driven by evidence given the limitations of establishing a diagnosis, the potential for practicing Cosmetic Endocrinology cannot be underestimated. With this, arises concerns that GH has the potential to be prescribed as a means for height augmentation in the absence of true patient pathology. The latter represents a complete diversion from its originally intended use which was the treatment for GHD. When used in this manner societal costs are tremendous and there is little return especially during the teenage years when the cost per inch of height gained progressively rises. The below figure summarizes the landmark events surrounding GH use which have led to the present ethical conundrum.
The ethical principles of beneficence, non-maleficence, autonomy, and justice serve as a framework to contextualize GH use in the era of its abundance where beyond being a treatment for true GHD, its use is as an expansive biotechnology, as put forward by Allen Buchanan. Such applies to the use of technologies/products beyond the original disease/indication for which they were intended. The distinction of GH being used an enhancement vs treatment is important. If used as enhancement, the question as to who pays for coverage is pertinent since medical necessity is the existing paradigm used by insurers to approve treatment. Hence, justice is at play, in determining the allotment of resources beyond what’s medically necessary.
Since GH is prescribed by a physician, usually seen as the gatekeeper of the health system, its use in the context of treatment for disease allows it to fit within the category of health care and this has implications. Since physicians and other patient care providers are often the first point of contact between patients and the medical system, these professionals may determine service allocation. If health care is seen as a right, then the lack of GH in some settings may raise the issue of a lack of justice in that resources may be unjustly allocated. So, the categorization of GH as a treatment vs enhancement and with the possibility of it being an expansive biotechnology, has ethical implications for access.
Regarding autonomy, prescribing GH to children without true GHD in the case of FDA approval for the indication of idiopathic short stature (defined by height less than 2.3rd percentile for age but with no actual cause), brings the issue of assent to the forefront of the discussion. In the absence of a pathologic condition such as GHD which necessitates treatment, should the child be allowed to have such therapy which arguably may not be medically necessary? Treatment in this context could be viewed as having the potential to shift what constitutes normalcy. In every population a certain number of individuals attain heights between -1 and – 2 standard deviations below the population’s mean height. When GH is used as an enhancement, there will be an erosion of these population statistics which are now considered as normal. This will blur the distinction between normalcy and what isn’t.
Pertaining to the ethical principles of beneficence and non-maleficence there’s some uncertainly regarding the long-term implications of GH supplementation in the absence of true pathology such as GHD although some authors believe that this is safe. The latter, view is however, debatable. Post marketing surveillance data acquired by the National Cooperative Growth Study (NCGS) utilized in the early phase of rhGH marketing are considered as incomplete, possibly lacking in data based on its reliance on physicians’ reporting and its inability to record events which develop after the initiation of treatment. Additionally, though results from observational studies such as NordiNet IOS and ANSWER show no adverse events related to long term GH use, the European SAGhE study indicated that though there was no increased all-cause mortality, there’s an increased mortality related to the circulatory and hematological systems. With these results, more continued long-term studies are needed.
Conclusion
Over the last six decades since the first recorded case of GH being used as treatment for GHD, its use has expanded to contexts which can be considered as non- medical. This broadened use has occurred within the ambits of conditions permissible to treatment by the FDA, such as idiopathic short stature. With this increased usage generated by increased product supply several ethical issues have arisen. Ultimately, medical professional should be cognizant that the decision to prescribe GH can be fraught with ethical issues which merit further consideration.
Author: Rohan K. Henry
Affiliation: The Ohio State Univerity College of Medicine/ Section of Endocrinology, Nationwide Children’s Hospital, Columbus, Ohio 43205, USA.
Competing interests: None declared