 Six weeks ago I discussed minimalism, two weeks ago the meaninglessness of “meaningful”, and last week “clinically meaningful”, the last of which really means “clinically important”. Now all of this comes together in the concept of a minimal clinically important difference.
Six weeks ago I discussed minimalism, two weeks ago the meaninglessness of “meaningful”, and last week “clinically meaningful”, the last of which really means “clinically important”. Now all of this comes together in the concept of a minimal clinically important difference.
The general idea of a clinically important difference is by no means new. Here it is, for example, in a 1949 paper [1]: “A clinically important difference may exist between the amount of accommodation which a patient will use to see clearly a near object when wearing contact lenses and when wearing spectacle lenses.” There are probably earlier instances.
In 1989 Gordon Guyatt and colleagues defined the minimal clinically important difference (MCID) as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient’s management” [2]. They showed how in some cases the minimal clinically important difference could be estimated from clinical trials, using patient questionnaires. Using a 7-point Likert scale to measure responses, they found that a within-subject change in score of 0.5 represented the minimal important difference in those cases. They also proposed that such information could be used when studying other patients and in planning new trials of interventions. Other methods have been described [3]. More recently, the minimal clinically important difference has been defined, more succinctly, as “The smallest difference a patient, or the patient’s clinician, would be willing to accept to use a new intervention” [4]. To which one might add “…given a favourable benefit to harm balance.”
However, there are earlier instances of the term, all of them in papers by Guyatt and his colleagues [5,6,7,8]. In the earliest, for example [5], they wrote that “… we suggest a new index of responsiveness to assess the usefulness of instruments designed to measure change over time. This statistic, which relates the minimal clinically important difference to the variability in stable subjects, has direct sample size implications.”
These earlier instances suggest that the term was already in use, perhaps even before 1987, although I have found no other examples. Nor have I have found instances before 1998 of variants, such as “minimal clinically relevant difference” or “minimal clinically meaningful difference”, the former in the European Commission’s “Rules Governing Medicinal Products in the European Union” and the latter in a book titled Osteoarthritis, edited by Brandt, Doherty, and Lohmander (OUP, 1998). Parkinson’s disease has also spawned the term “minimal clinically relevant incremental difference”, the earliest instance being in 2003 [9].
In 1994, Guyatt and his colleagues suggested that the word “clinically” could be omitted from MCID, since “this terminology focuses attention on the clinical arena rather than patients’ experience in their day-to-day lives” [10,11]. They defined the minimal important difference (MID) as “the smallest difference in score in the outcome of interest that informed patients or informed proxies perceive as important, either beneficial or harmful, and which would lead the patient or clinician to consider a change in the management” [12].
In advance of a study you might ask patients how much anticipated benefit they would consider worthwhile, for example how many extra months of good-quality life in the treatment of a terminal illness. That estimate could be considered to be the minimum desirable incremental 50% survival time. The size of trial needed to demonstrate whether an intervention produced the estimated MID could then be calculated in the usual way, with selected type 1 and type 2 errors. I suspect, however, that anticipated effect sizes are often back-calculated, using estimates of the numbers of subjects that investigators think they are likely to be able to recruit.
Although the concept of the MCID has been around since the 1980s, it was not until 2010 that its use started to increase, and even now the total number of papers in which the term has even been mentioned is relatively small (Figure 1). It is certainly not clear how often the minimal clinically important difference has been calculated from reported data [13].
Perhaps we need an online catalogue of minimal clinically important differences.
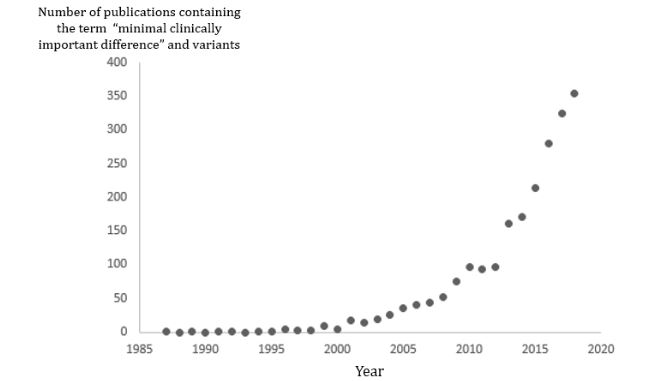
Figure 1. The numbers of papers containing the term “minimal clinically important difference” and variants (e.g. “change” or “improvement” instead of “difference”) since 1987 (source PubMed); plotting the data as a ratio of all publications gives a graph with a very similar shape
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.
References
- Alpern M. Accommodation and convergence with contact lenses. Am J Optom Arch Am Acad Optom 1949; 26(9): 379-87.
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10(4): 407-15.
- Cook CE. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): a necessary pretense. J Man Manip Ther 2008; 16(4): E82-3.
- Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017; 18(1): 122.
- Guyatt G, Walter S, Norman G. Measuring change over time: Assessing the usefulness of evaluative instruments. J Chronic Dis 1987; 40(2): 171-8
- Guyatt GH, Townsend M, Pugsley SO, Keller JL, Short HD, Taylor DW, Newhouse MT. Bronchodilators in chronic air-flow limitation. effects on airway function, exercise capacity, and quality of life. Am Rev Resp Dis 1987; 135(5): 1069-74.
- Guyatt GH, Townsend M, Kazim F, Newhouse MT. A controlled trial of ambroxol in chronic bronchitis. Chest 1987; 92(4): 618-20.
- Guyatt GH, Sullivan MJ, Fallen EL, Tihal H, Rideout E, Halcrow S, Nogradi S, Townsend M, Taylor DW. A controlled trial of digoxin in congestive heart failure. Am J Cardiol 1988; 61(4): 371-5.
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003; 18(7): 738-50.
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol 1994; 47(1): 81-7.
- Schünemann HJ, Guyatt GH. Commentary—goodbye M(C)ID! Hello MID, where do you come from? Health Serv Res 2005; 40(2): 593-7.
- Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). COPD 2005; 2(1): 81-9.
- Johnston BC, Ebrahim S, Carrasco-Labra A, Furukawa TA, Patrick DL, Crawford MW, Hemmelgarn BR, Schunemann HJ, Guyatt GH, Nesrallah G. Minimally important difference estimates and methods: a protocol. BMJ Open 2015; 5(10): e007953.
Acknowledgements: Thanks to Carl Heneghan and David Nunan for helpful comments.
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.
