 To recap. Medicines are given International Nonproprietary Names (INNs) by an expert panel of the World Health Organization, using principles that are not uniformly adhered to. When the names are confusable medication errors can occur.
To recap. Medicines are given International Nonproprietary Names (INNs) by an expert panel of the World Health Organization, using principles that are not uniformly adhered to. When the names are confusable medication errors can occur.
Consider monoclonal antibodies (mAbs). Apart from the first, muromonab, they all end in the stem -mab. Substems are formed by adding a penultimate syllable, indicating the species of origin, and an antepenultimate syllable, which vaguely indicates the target. Thus, the tripartite substems do not specify the pharmacological actions of the antibodies, which is what stems and substems are supposed to do. The system is expanding all the time, as new infixes are invented and old ones fall into desuetude. Here is the latest version, as far as I can determine, old and new included, and below are examples of how the system works.
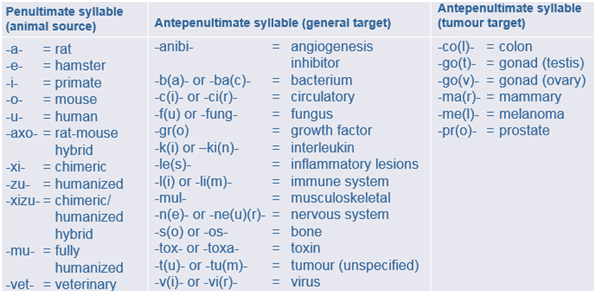
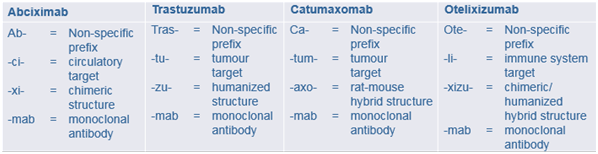
Systematic though these names are, they are not always appropriate. The pharmacological target for abciximab, for example, is platelet IIb/IIIa receptors, not a cardiovascular circulatory element. And the name “catumaxomab” does not tell you that it is not a simple monoclonal antibody with a single target, but a bispecific trifunctional one.
There are currently 346 mAbs in the WHO’s list of INNs; 35 of them end in -ximab, 35 end in -mumab, and 138 end in -zumab; 50 end in -lizumab. The potential for confusion is obvious. Consider, for example, ruplizumab, siplizumab, and teplizumab, with their different targets and proposed clinical uses.
And so to biosimilars also called “follow-on biologics” or “similar biotherapeutic products (SBPs)”. They are defined by the European Medicines Agency (EMA) as biological medicines that are similar to biological medicines that have already been authorised for use. Made by or derived from biological sources, they can be relatively small molecules, such as human insulin or erythropoietin, or complex molecules such as monoclonal antibodies.
In the 1980s, when it became clear that recombinant forms of compounds such as erythropoietin, follitropin, and interferon made by different manufacturers were differently glycosylated, the WHO introduced a naming scheme that involved adding a Greek letter to the name: follitropin alfa, beta, gamma; epoetin alfa, beta, theta, zeta. In a minor variant on the theme, epoetin alfa was also introduced into Australia as epoetin lambda. One version of epoetin was so highly glycosylated that it was given a different name altogether, darbepoetin.
The problem is that one cannot be sure that two biosimilars will have the same beneficial effects or that they will not cause different harms. For example, when I looked in Pubmed for reports of red cell aplasia in patients taking epoetins, I found 90 reports out of a total of 2124 papers on epoetin alfa (4.24%) and 45 reports out of 537 papers on epoetin beta (8.38%). Now this is a crude test, and it does not show that epoetin beta is more likely to cause red cell aplasia than epoetin alfa, but it raises the question, and a formal study would be of interest.
In the EU biosimilars may not be marketed until the period of data exclusivity of the original biologic has expired, but many biologics are now coming to that point. An estimated 30 biologics with combined sales of US$51bn came off patent in 2015. Thus, the task of naming biosimilars has become crucial, and different countries have different approaches.
In the US, for example, the preferred method is to add the name of the manufacturer (for example, “filgrastim-sndz”); in Australia the preferred method has been to add a code composed of “sim” plus a random three letter identifier. In 2014, therefore, the WHO proposed a voluntary scheme in which all biologics—except vaccines and complex biologically extracted proteins, such as heparin—would be given a “biological qualifier”, a random four letter code and an optional two digit check sum, tied to the place of manufacture. Using non-repeating consonants (omitting the vowels and y and strings that are already abbreviations, such as MRCP and FRCS) would yield over 110 000 possible codes, more than enough. The system, the final version of which was issued in January, is highly controversial, partly because the codes are meaningless. It seems unlikely that there will be international participation.
In the meantime, the advice on prescribing biologics that may have biosimilars is to use the brand name of your preferred product.
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.
