 To recap: over the past three weeks I have used the metaphor of crossing bridges in discussing translational research in clinical medicine; derived a model of it from definitions in the Cooksey report; and, recognising its weaknesses, modified the model based on later descriptions.
To recap: over the past three weeks I have used the metaphor of crossing bridges in discussing translational research in clinical medicine; derived a model of it from definitions in the Cooksey report; and, recognising its weaknesses, modified the model based on later descriptions.
However, the second model still has weaknesses. For example, it retains the false dichotomy between basic and applied research and does not take into account the fact that the processes involved have effects in both directions, although some have included two way arrows in similar models. Furthermore, it does not take account of factors that influence the use of interventions in clinical practice, including the benefit to harm balance, cost effectiveness, and the need to monitor outcomes. A more complete model is illustrated in the figure below.
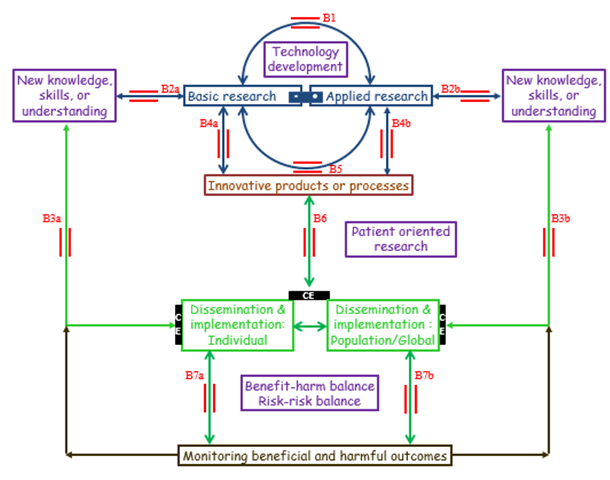
Figure An operational model of translational research, recognising the interrelations of the different components of the model and the two way processes (bridges in red) that link them, the barrier of cost effectiveness (CE, black bars), and the importance of predicting and monitoring beneficial and harmful outcomes
• Bridge B1: the interplay between basic and applied research, without necessarily generating innovative products or processes
• Bridges B2a and B2b: whereby basic and applied research lead to new knowledge, skills, or understanding
• Bridges B3a and B3b: direct applications in individuals, discrete populations, or globally
• Bridges B4a, B4b, and B5: The emergence of innovative products or processes, providing opportunities for further research, dissemination, including education, and implementation
• Bridge B6: patient oriented research, leading to dissemination and implementation of innovative products or processes, and to new insights that require further research, basic and/or applied
• Bridges B7a and B7b: use of the technology in individuals, subject to a favourable benefit/harm balance and risk/risk balance and proper monitoring of outcomes; an unfavourable benefit/harm balance or risk/risk balance can suggest further modifications
In this model, seven bridging sets of bidirectional arrows (B1 to B7) replace the 6 steps (T0 to T5) of the previous model. They illustrate the different ways in which research can lead to clinical applications and how those applications can stimulate further research. Bridge B1 is the interplay between basic and applied research, without necessarily generating innovative products (e.g. medications and devices) or processes (e.g. ways of administering or applying them), but leading to new knowledge, skills, or understanding (bridges B2a and B2b), which can be applied directly in individuals, populations, or globally (bridges B3a and B3b).
Both basic and applied research, either by themselves (bridges B4a and B4b) or in combination (bridge B5) can lead to innovative products or processes, which can in turn be disseminated and implemented (bridge B6). In turn, innovative products and processes may generate opportunities for further research (bridges B4a and B4b), and implementation can lead to new insights that require further research, basic and/or applied. If the benefit/harm balance and the balance of risks of treating and not treating (the risk/risk balance) are favourable in the restricted population that has been studied in the early phases of development, the technology can be used in individuals, but then its effects both in individuals and in the population as a whole must be monitored for beneficial and harmful outcomes (bridges B7a and B7b). An unfavourable benefit/harm balance or risk/risk balance can suggest further modification.
This model includes two other features of research in relation to healthcare that earlier models neglect: (a) a bar linking basic and applied research, implying that they form a continuum not a dichotomy, and (b) the recognition that cost utility or cost effectiveness analyses (black bars) are necessary in judging whether to implement a technology in clinical practice.
This model has several implications.
- The processes are non-linear, contrary to earlier models of scientific research.
- The model is of necessity non-deterministic, in the sense that it does not specify the structures of the individual bridges it involves; for each specialism different structures will be required.
- No single aspect of research is a standalone activity, of itself and for itself. Each has to take into account the outcomes that flow from implementation of the results, which generally then suggest further research. Even research that was never intended to have practical relevance (“blue skies” or “curiosity driven” research) may have outcomes that will drive further (e.g. practical) research, and practical outcomes may suggest further blue skies research.
- Too rapid implementation of an innovation or an idea that has not been thoroughly tested and shown to be desirable, effective, safe (i.e. non-harmful), and cost effective can be hazardous, as can failure to monitor an intervention that has been implemented.
- Finally, it is clearly not possible for any one individual or discrete group of individuals to carry out translational research. Even a single large institute intended to be devoted to translational research could not possibly encompass all the skills and facilities required.
This last point demonstrates that translational research is something to which everybody can contribute, not merely those who call themselves translational researchers. Every outcome, every piece of evidence has the potential of contributing to the whole edifice.
Next week I shall propose definitions of translational research and of research itself.
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.
