In the October issue of JNNP, Lin and colleagues explore plasma levels of a-synuclein as a biomarker in patients with varying stages of Parkinson’s disease (PD), across both motor and non-motor domains.The extensive evaluation of biofluids in the hunt for diagnostic and prognostic biomarkers in PD has been disappointing and largely inconclusive to date. Studies have mainly focused on CSF alpha (a)-synuclein levels, as this is the major misfolded cortical protein critical to PD pathogenesis, aggregated within Lewy bodies (Figure 1). However, in addition to the invasive nature of CSF testing, the risk of blood contamination affecting outcomes and technical limitations across labs, CSF a-synuclein has not emerged as a robust stand-alone biomarker in recent meta-analyses. Consequent exploration for more accessible biomarkers in the blood (serum or plasma) has also been difficult due to relatively low concentrations of a-synuclein in plasma, making this a challenge to accurately detect using standard immunoassay techniques. Prior studies have thus produced contradicting results.
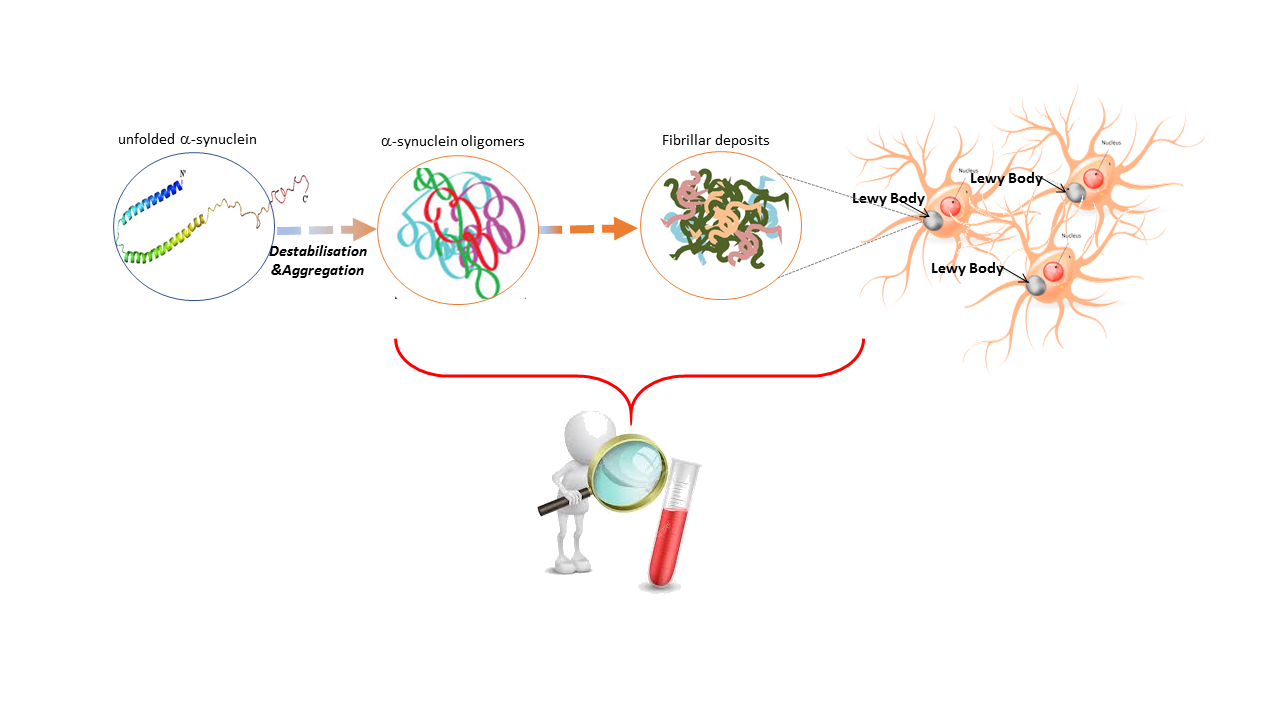
In this study, Lin et al employ a novel immunomagnetic reduction (IMR) based immunoassay to quantitate plasma a-synuclein levels in 80 patients with PD (compared to 34 healthy controls). By utilising magnetic nanoparticles to bind biomolecules, the IMR assay is able to detect and quantify a-synuclein at the fg/mL level with high sensitivity and accuracy. Overall, the authors reported significantly higher plasma a-synuclein levels in PD patients compared to controls. Although no correlation between plasma levels and motor severity was found, a significant negative correlation between plasma a-synuclein levels and MMSE scores was seen after correcting for age, gender and disease duration. This clinically translates to higher levels of plasma a-synuclein in patients with more severe cognitive dysfunction compared to patients with mild cognitive impairment or normal cognition.
The promising possibility of an accessible blood biomarker to detect cognitive dysfunction in PD patients is of unquestionable value, particularly given the debilitating nature of this non-motor symptom and the conferred burden of disease on caregivers. Although limited by its cross-sectional design, this study and its use of novel IMR immunoassays establishes the technical platform required for further longitudinal study. With more detailed neuropsychological testing (particularly in cognitive domains) in a larger group of patients, prediction of future cognitive decline in PD has now become a tangible possibility, with major implications for earlier detection and targeted multidisciplinary interventions.