This is the sixth blog by Dr Ammara Hughes on Primary Care Leadership and COVID vaccination. Read the first, second, third, fourth, and fifth blogs in the series.
Drugs are a funny old thing. One minute, they’re the best thing since sliced bread. The next, you’d rather be sticking hot needles in your eyes than ever consider taking them.
In my 25 years so far in medicine, there are many such tales to tell.
Take the most common long term condition we see as GPs. Hypertension. I cannot tell you how many times with the same patients, I’ve had to change them from one drug, to another, only a few years later to change them back again.
Aspirin
When I first became a GP, we were advised to put all people with hypertension on aspirin to prevent heart attacks. As years went by, it became clear that aspirin was beneficial in patients with a history of a heart attack, but all that aspirin did in healthy people was increase the risk of a gastrointestinal bleed. That doesn’t mean aspirin shouldn’t be used at all. In fact, it remains an incredibly safe and effective drug in many clinical scenarios.
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)
ALLHAT was an 8 year long, multicentred, randomized, double-blind controlled trial (1). The words every medical statistician wants to hear. Published in the Journal of the American Medical Association (JAMA) in 2002, telling us that thiazide diuretics were by far the best drugs for hypertension to prevent heart attacks in preference to ACE inhibitors or calcium channel blockers.
So we diligently put everyone on a thiazide. Lots of letters and conversations with patients. Many, many appointments to discuss blood tests and side effects and then check blood pressure when changing from one drug to another.
Lo and behold, a few years later, review of the same data cast doubt on the conclusions and we were then told everyone should be on an ACE inhibitor or a calcium channel blocker.
The narrative, however, aside from the science, suggested there was more at play underlying the recommendations. Michael Weber, in his 2007 commentary on the ALLHAT report, wrote in the Journal of Clinical Hypertension (2):
“Because hypertension is such an aggressive marketplace, several of the newer branded drugs are priced competitively and allow physicians reasonable flexibility in choosing treatment based on therapeutic need. Most importantly, we all now recognize that effective blood pressure control in most hypertensive patients calls for logical drug combinations that typically will include all the drug types examined in ALLHAT as well as other classes.”
The rise and fall of Astrazeneca
It was only a matter of time that such scrutiny was placed on the COVID vaccines. Astrazeneca’s vaccine has become the unwitting star of this particular show.
Our contribution to the Astrazeneca story began on 7 January 2021. We found ourselves in the midst of a high profile launch, while we were busy with a Pfizer clinic.
We had eyes only for Pfizer. The Health Secretary, however, had an early start to see Astrazeneca, the home grown vaccine, in action on its first day.
The issue was my team didn’t quite get that brief. We were delighted to showcase our vaccine hub and give our team and patients the opportunity to tell the Health Secretary how it was going and what we might be able to do better.

“So this is Pfizer?” the Health Secretary enquired as Raj Gill, lead physician associate for our vaccine hub administered a vaccine.
“Yes,” I replied. “Our Astrazeneca hasn’t arrived yet.”
The subsequent news story became about us not having Astrazeneca for the Health Secretary’s visit. Nevertheless, everyone heard about Astrazeneca that day. Our delivery when it arrived later, was major news.
9 January 2021, we kept Astrazeneca in the headlines by vaccinating Dame Joan Collins (Raj Gill again doing the honours). Dame Joan helped spread vaccine positivity as an early recipient. We had no problem filling our Astrazeneca clinics after two high profile visits within a week.
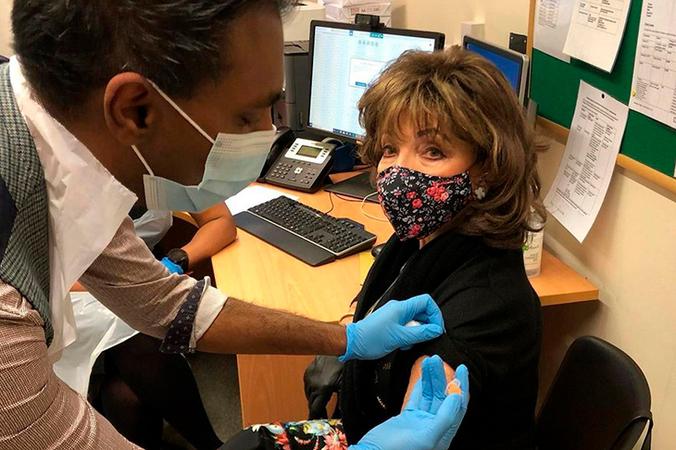
Joan Collins, posted with permission
Astrazeneca was in high demand. Doses were being requested faster than supplies could be produced across the world. Amidst all this, however, Europe was raising concerns about the possible link between rare blood clots and the Astrazeneca vaccine.
By 15 March 2021, many EU countries had temporarily suspended its use. The regulators at the time had made no such recommendation, and it was business as usual in our vaccine hub and satellite clinics. As health professionals, we have to look past the possibility of politics and business as factors, and make sure we are in possession of facts that we can relay to the public objectively and calmly.
Steady state
It is inevitable that any new drug on the market will have emerging side effects or risks. Our role as health professionals is to report adverse events, remain appraised of the risks and act in the best interests of our population.
The evidence to date still overwhelmingly supports the use of Astrazeneca’s COVID vaccine for our mass vaccination programme. We have yet to see how the newer vaccines on the market will fare against it.
The rise and fall, and persistence, of many therapeutic agents in the last century would suggest that all COVID vaccines, including Astrazeneca, will remain part of our armoury for years to come.
References
[1 ]The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or CCB vs. diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002; 288: 2981– 2997.
[2] Weber, MA . The ALLHAT report: a case of information and misinformation. J Clin Hypertens (Greenwich). 2003;5(1):9–13.

Dr Ammara Hughes
Dr Ammara Hughes MBBS MRCGP (2004) is a GP partner at Bloomsbury Surgery, Central London, and co-Clinical Director, Central Camden PCN and a member of the NHS Confederation PCN network. She qualified as a doctor from Charing Cross and Westminster Medical School in 1996. She spent 8 years in hospital medicine in London, before becoming a GP in 2004. She has been in leadership in the NHS since 2007. She was an elected Governing Body GP member of Camden Clinical Commissioning Group from 2011-2017, serving two terms. Since then, she has undertaken provider lead roles. She was Vice Chair of Camden Health Evolution from 2017-2019, stepping down to take on the role of Clinical Director of Central Camden Primary Care Network.
Declaration of interests
I have read and understood the BMJ Group policy on declaration of interests and declare the following interests: none.