The buzz-word of evidence informed decision making is all pervasive in the health policy and systems space. However, it is easier said than done. To facilitate evidence informed decision making, the Government of India established a formal institutional structure known as Health Technology Assessment in India (HTAIn) under Department of Health Research (DHR) in 2016-171–3. We share our experience around the process of establishment, development and evolution of HTA in India. All the way we felt that in order to make the HTA system successful in any country, it must emerge as an inclusive model.
The primary role of HTAIn is to assess health technologies in terms of their cost, effects, equity, feasibility and budgetary implications as and when a demand for such a study comes from a user department. User department for HTAIn could be a decision maker who use evidence informed studies at Central or State Government level. Whenever a country initiates to recruit a new assessment system like HTA in their health system, it also brings about a lot of fear of uncertainty and dilemma amongst the key stakeholders. The HTA body may be apprehensive about the acceptance of the recommendations it provides or any backlash generated due to the lack thereof, by its industry partners. The apprehension may be due to the fact that it is a fairly new assessment system. The industry partners may have a concern that they will now be asked to go through another level of regulation. Many questions may arise due to this. Will they be held liable if they don’t stick to the HTA recommendations? Will this lead to any price capping of technologies assessed? The aftermath of all this uncertainty and confusion among the two groups may leave Government and other decision makers in a difficult position. Having said that, this situation resolves with time and with active involvement of stakeholders in the HTA process, resulting in transparency.
HTA in India has observed that root cause of this fear of uncertainty originates from the lack of communication between the core groups of the HTA stakeholders i.e., HTA body, decision makers, industry partners and patients. This may also be accounted for by the lack of involvement of industry partners at various stages of conducting an HTA study. To avoid any fear and confusion and to keep the process transparent, HTAIn has taken steps to include stakeholders’ consultation for each HTA study a mandatory step in its process manual8.
For each HTA study, HTAIn conducts at least two stakeholders’ consultation meetings, one at the time of developing the study proposal and the other at the time of completing the study to keep all the stakeholders informed about each step of the study. For these consultation meetings, utmost care is taken to invite all concerned stakeholders from varying fields including industry partners, Central as well as State Government officials, researchers from the field of study, clinicians and patient association groups, etc8.
Another important aspect for HTA system is transparency at all levels. HTAIn has made clear policies to maintain the transparency. Before even taking up its first HTA study, HTAIn has prepared a process manual documenting the key steps of the HTA process to be followed. A guidance document to guide the economic evaluation studies has also been prepared by HTAIn. Key documents like HTAIn manual and the HTAIn compendium also aimed to maintain the transparency and inclusiveness. Continuous review of all technology assessments by an unbiased technical appraisal committee is another important way HTAIn has adopted to enhance the transparency in the system. HTAIn has made available its key documents, study outcome reports, meeting discussion points, working groups and policy briefs on its official website9. A national HTA database is under process that will include details of all the studies been completed or under process under HTAIn10. The database will be made available on HTAIn’s website.
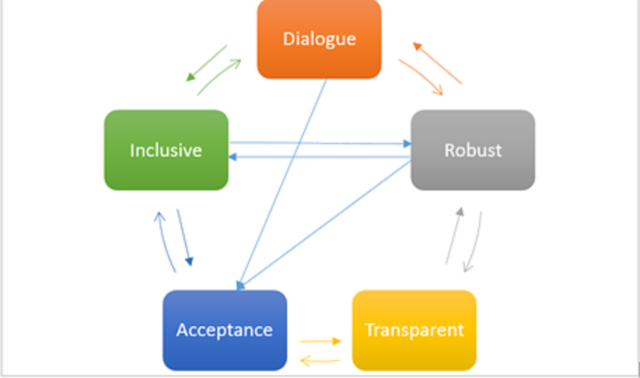
A robust HTA system is the one which follows set national and international standards while conducting studies of various nature, for example, costing study, quality of life study, systematic reviews, etc.11,12. Another process of importance is to conduct quality assurance by using established checklists or modifying them to suit country specific HTA evaluation needs11.The HTA body should maintain their autonomy in approving and disapproving the study outcomes and recommendations, avoiding any kind of influence by any third party.
The HTA process has already proven itself to be valuable in ensuring Governments’ objective of delivering a safe, effective and efficient health care system in various countries around the globe who have long adopted it13. These include, the UK, Canada, Australia, some parts of Europe and Thailand to name a few13–18. HTA may prove to be even more useful in a country like India where the population is huge and judicial allocation of resources is the only way to go forward. This pertains more applicable at this hour when the Government of India is stepping towards achieving Universal Health coverage19.
To conclude, the process of HTA development must be interactive, inclusive, transparent and robust. A successful system will be the one where all stakeholders can provide inputs freely with a belief that their inputs will be considered seriously. It should involve all relevant stakeholders who are affected by the given study outcome to be called inclusive. All details of a study should be publicly available including study methods so they can be validated if required and is also reproducible. This should be a robust process which is credible and unbiased and follows the international quality standards.
About the Authors :
Shalu Jain and Kirti Tyagi are scientists in the Health Technology Assessment India, Dept. of Health Research,
Ministry of Health and Family Welfare, Government of India.
Competing Interests: None declared
References:
- Prinja S, Downey LE, Gauba VK, Swaminathan S. Health Technology Assessment for Policy Making in India: Current Scenario and Way Forward. PharmacoEconomics – Open. 2018;2(1):1-3. doi:10.1007/s41669-017-0037-0
- Dabak SV, Pilasant S, Mehndiratta A, et al. Budgeting for a billion: applying health technology assessment (HTA) for universal health coverage in India. Heal Res Policy Syst. 2018;16(1):115. doi:10.1186/s12961-018-0378-x
- Downey LE, Mehndiratta A, Grover A, et al. Institutionalising health technology assessment: establishing the Medical Technology Assessment Board in India. BMJ Glob Heal. 2017;2(2):e000259. doi:10.1136/bmjgh-2016-000259
- Neumann PJ. Lessons for Health Technology Assessment: It Is Not Only about the Evidencev he_558 45..47. Value Heal. 2009;12:S45-S48. doi:10.1111/j.1524-4733.2009.00558.x
- Nielsen CP, Lauritsen SW, Kristensen FB, Bistrup ML, Cecchetti A, Turk E. Involving stakeholders and developing a policy for stakeholder involvement in the European network for health technology assessment, EUnetHTA. Int J Technol Assess Health Care. 2009;25(SUPPL.S2):84-91. doi:10.1017/S0266462309990729
- Gagnon M-P, Desmartis M, Gagnon J, et al. Introducing the patient’s perspective in hospital health technology assessment (HTA): the views of HTA producers, hospital managers and patients. Heal Expect. 2014;17(6):888-900. doi:10.1111/hex.12010
- Goodman CS, Ahn R. Methodological approaches of health technology assessment. Int J Med Inform. 1999;56(1-3):97-105. doi:10.1016/S1386-5056(99)00049-0
- Health Technology Assessment in India (HTAIn) – Process Manual. https://htain.icmr.org.in/index.php/documents/publications/process-manual. Accessed February 9, 2020.
- Health Technology Assessment in India (HTAIn) – Health Technology Assessment in India (HTAIn). https://htain.icmr.org.in/. Accessed February 9, 2020.
- Jain S, Rajshekar K, Sohail A, Gauba V. Department of Health Research-Health Technology Assessment (DHR-HTA) database: National prospective register of studies under HTAIn. Indian J Med Res. 2018;148(3):258. doi:10.4103/ijmr.IJMR_1613_18
- Hailey D. Toward transparency in health technology assessment: A checklist for HTA reports. Int J Technol Assess Health Care. 2003;19(1):1-7. doi:10.1017/S0266462303000011
- Drummond MF, Schwartz JS, Jönsson B, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24(3):244-258. doi:10.1017/S0266462308080343
- O’Donnell JC, Pham S V., Pashos CL, Miller DW, Smith MD. Health technology assessment: Lessons learned from around the world – An overview. Value Heal. 2009;12(SUPPL. 2):S1-5. doi:10.1111/j.1524-4733.2009.00550.x
- Woolf SH, Henshall C. Health technology assessment in the United Kingdom. Int J Technol Assess Health Care. 2000;16(2):591-625. doi:10.1017/S0266462300101175
- Hailey D. The history of health technology assessment in Australia. Int J Technol Assess Health Care. 2009;25(SUPPL.S1):61-67. doi:10.1017/S0266462309090436
- O’donnell JC, Pham S V, Pashos CL, Miller DW, Smith MD. A Perspective on the Use of Health Technology Assessment in the United Kingdom”; “Health Technology Assessment in Canada: 20 Years Strong?”; Health Technology Assessment: A Perspective from Germany”; “Health Technology Assessment: Reflections from the Antipodes”; and. Heal Technol Assess Heal Outcomes Res. 2009:1098. doi:10.1111/j.1524-4733.2009.00550.x
- Teerawattananon Y, Tantivess S, Yothasamut J, Kingkaew P, Chaisiri K. Historical development of health technology assessment in Thailand. Int J Technol Assess Health Care. 2009;25(SUPPL.S1):241-252. doi:10.1017/S0266462309090709
- Sorenson C, Chalkidou K. Reflections on the evolution of health technology assessment in Europe. Heal Econ Policy Law. 2012;7(1):25-45. doi:10.1017/S1744133111000296
- About Pradhan Mantri Jan Arogya Yojana (PM-JAY) | Ayushman Bharat I National Health Authority | GoI. https://pmjay.gov.in/about-pmjay. Accessed February 9, 2020.