Blog entry written on: Combination fixed‐dose beta agonist and steroid inhaler as required for adults or children with mild asthma: a Cochrane Systematic Review, (bmjebm-2021-111764)’
Authors: Iain Crossingham, Sally Turner, Sanjay Ramakrishnan, Anastasia Fries, Matthew Gowell, Farhat Yasmin, Rebekah Richardson, Philip Webb, Emily O’Boyle,Timothy Stopford Christopher Hinks.
Blog entry written on: Combination fixed‐dose beta agonist and steroid inhaler as required for adults or children with mild asthma: a Cochrane Systematic Review (bmjebm-2021-111764)
Is it time to rethink how we treat the majority of people with asthma? The findings of a new Cochrane meta-analysis of suggest that as-required use of combination fixed‐dose beta agonist and steroid inhaler is an effective and safe approach to treating adults with mild asthma, and could reduce the number of severe asthma attacks. This study lends support to changes to guidelines away from the use of short acting beta2-agonists alone in mild asthma. Such changes are being considered in countries such as the UK, and in some cases have already been implemented, such as in the international GINA guidelines.
Asthma affects 1:12 adults in the UK and 350 million people worldwide, the majority of whom have mild disease. Most treatment is with two types of inhaler. ‘Reliever’ inhalers treat just the symptoms by relaxing the constricted airways, these medicines are ‘beta2-agonists’, some of which are fast-acting (FABA) and relieve symptoms within 5-10 minutes. ‘Preventers’ are inhaled corticosteroids (ICS), which treat the underlying inflammation. ICS are very effective in reducing severe attacks of asthma and asthma deaths, but need to be taken for several days before benefits are felt.
Until recently mild asthma has often been treated with just a FABA alone, like salbutamol, relieving symptoms, but not reducing inflammation, and leaving patients at risk of serious exacerbations. Often patients do not recognise increasing symptoms as a warning of dangerous airway inflammation and an urgent need to take more preventer ICS. An alternative approach – prescribing regular, daily ICS in mild asthma – should be safer but because these medicines take several days to take effect, often people with mild asthma fail to use them regularly. Over-reliance on symptomatic relief from FABAs is often identified as a tragic cause of preventable asthma deaths, for instance by the UK’s National Review of Asthma Deaths.
A new approach has been proved very effective in moderate or severe forms of asthma where a single inhaler contains both the beta-agonist and the inhaled corticosteroids in a single device. Until now it was not known whether these inhalers, just used as-required, might also be an effective way to treat people with mild asthma.
Our team of Cochrane researchers in East Lancashire and Oxford assessed data from recent, large, randomised-controlled trials (RCTs) addressing this question in adults with mild asthma.
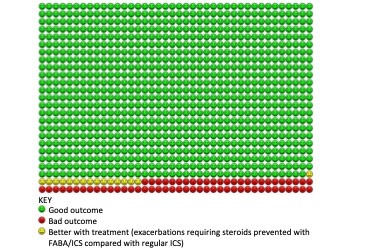
We found 5 RCTs enrolling 9657 participants, from large multinational studies performed in 6 continents. The results were clear. In the first comparison we found high-certainty evidence that symptom-driven, as-required FABA/ICS compared with reliever-only treatment reduced severe exacerbations and rates of emergency hospital admissions with asthma. In practice over one year this would be equivalent to 109 people out of 1000 with mild asthma in the FABA alone group experiencing a severe exacerbation, compared with just 52 people using FABA/ICS as-required. We would also expect only 1/3 of the number of hospital admissions with asthma (odds ratio 0.35).
In the second comparison, as-required use of FABA/ICS compared with regular daily ICS led to similar rates of severe exacerbations but fewer hospital admissions and lower overall ICS dose. These findings were based on lower-certainty evidence because two of the trials were open-label, there was heterogeneity between trials, and event rates were relatively low.
Several key questions remain:- how cost effective is this approach; what are longer-term outcomes; can this approach be used in children; and how could these findings translate to low-to-middle income countries?
For now, it seems clear that FABA/ICS are significantly safer than FABA alone for adults with mild asthma, and good outcomes are achieved with as-required FABA/ICS when used as first line treatment in mild asthma.
Author
Timothy Hinks FRCP PhD
Institution: Respiratory Medicine Unit, University of Oxford
Conflict of interest statement: I Crossingham has been involved in recruitment for a GlaxoSmithKline-sponsored trial of inhaled nemiralisib for COPD, but did not directly receive funding for this. S Turner reports money for travel from Novartis in 2019 for an educational event. S Ramakrishnan is undertaking a PhD supported by an unrestricted research grant from AstraZeneca. He has attended educational events sponsored by AstraZeneca (2019). TSC Hinks has received research funding from the Wellcome Trust, NIHR, the Beit Guardians; has received speaker fees from AstraZeneca, Boehringer Ingelheim; his research team have received funding from Sanofi. The other authors declare they have no relevant conflicts of interest.
DISCLAIMER
The views and opinions expressed on this site are solely those of the original authors. They do not necessarily represent the views of the BMJ and should not be used to replace medical advice. All information on this blog is for general information, is not peer-reviewed, requires checking with original sources and should not be used to make any decisions about healthcare. No responsibility for its accuracy and correctness is assumed by us, and we disclaim all liability and responsibility arising from any reliance placed on such commentary or content by any user or visitor to the Website, or by anyone who may be informed of any of its content. Any reliance you place on the material posted on this site is therefore strictly at your own risk.