Marisa de Andrade & Gerard Hastings
Institute for Social Marketing, University of Stirling
Recent discussions about an endgame for tobacco have built on a sense that we in the tobacco control (TC) movement know where we are going. The consistent application of evidence based strategies, from adbans to tax increases, was, it seemed, driving an inevitable progression towards a smoke-free world; the only question was ‘when would the prevalence line cross the X axis?’
Tobacco harm reduction (THR) has been a carefully modulated dimension of this debate. Now this balance has been unsettled by the sudden arrival onto the market of a wide range of e-cigarettes and other Nicotine Containing Products (NCPs). The development suggests both opportunities (such as greatly reduced harm for the heavily addicted) and threats (like the potential rehabilitation of the tobacco industry), and in the process throws up multiple research questions. This THR Research Agenda commissioned by Cancer Research UK presents a first attempt to map these questions. It was informed by a review of the academic and grey literature and consultations with twelve TC experts.
It quickly became apparent that research questions could be grouped into four broad (and sometimes overlapping) areas: impacts on the individual; the tobacco control movement; the political environment; and philosophical issues.
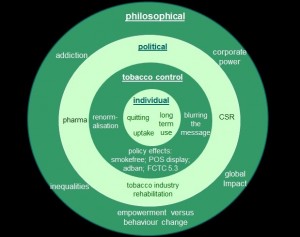
A taxonomy of harm reduction research
Individual
According to the National Institute of Health and Care Excellence (NICE) draft HR guidance, e-cigarettes offer a cleaner vehicle for the delivery of nicotine as ‘the harm associated with cigarette smoking is almost entirely caused by the toxins and carcinogens found in tobacco smoke’. Ongoing evaluations of safety (short and long-term) are recommended by the health body, a call which a recent German Cancer Research Center report and presentation to the European Parliament also makes very strongly.
This research needs to be balanced with an examination of efficacy: how effective are NCPs and e-cigarettes as smoking cessation aids and at helping smokers cut down, and what impact does this have on quitting? We also need to know how NCPs are being used – for dual use, temporary abstinence, long-term as a tobacco substitute or part of a quit attempt – and by whom, covering age, socio-economic status, gender and ethnicity. By extension, potential impact on individual and population level inequalities also needs to be assessed.
More broadly there is a need to examine how key stakeholders, including smokers, non-smokers, policymakers, primary healthcare staff, journalists, children and young people, are perceiving HR, NCPs and all related commercial and social marketing activity.
Tobacco control
Developments in THR and e-cigarettes also raise strategic questions for tobacco control. What priority should the TC community give to HR relative to other tobacco control approaches? We need to know how, if at all, HR interacts with these other approaches and specifically with complete cessation (eg do NCPs and/or e-cigarettes help or hinder quit attempts?) and youth prevention (eg could they act as a gateway to smoking?). In each case the net needs to be thrown wide to capture the effects not just of the products themselves, but the way they are presented and promoted in digital and conventional media.
The regulatory response is varying around the world and the efficacy and wider impact of these alternate models needs to be examined. In the UK, for example, where NCPs could soon be regulated by the Medicines and Healthcare products Regulatory Agency (MRHA), questions arise about how marketing will be overseen. What would the official channels be for reporting potential breaches, and how would this fit within the regulator’s remit? How well does this regime operate compared with other jurisdictions where tighter controls (eg complete adbans) are in place? More broadly, how did these different approaches arise?
The impact, if any, of HR and the use of NCPs on the denormalisation of smoking is currently unknown. Does e-cigarette use, for example, model smoking? More specifically, to what extent, if at all, do the new products undermine smoke-free legislation, or the packaging, point of sale (POS) display and advertising of them undermine tobacco marketing controls? Potential conflicts with current regulation could also raise concerns relating to the Framework Convention on Tobacco Control (FCTC). Specifically, how should Article 5.3 be interpreted and deployed when the tobacco industry (TI) is investing so heavily in HR and associated products?
Political
The FCTC also raises political debates. Is the TI using HR to engage in and influence health policy, perhaps via third parties (either commercial or public)? What, if any, conflict of interest does TI investment in reduced risk products present? Could it, for instance, undermine or remove public health gains from HR? And what is the TI’s business strategy with regard to NCPs and the implications for TC? Under what circumstances, if at all, could the TI come to be seen as a legitimate stakeholder? Is the tobacco TI using HR as a corporate social responsibility or stakeholder marketing strategy? If so, how is this happening and what are the potential dangers?
It is also necessary to know how research on HR and NCPs is being funded, and what impact will this have on TC. What, if any, similarities are there between TI interest in HR and its past activities around filtered, safe and low tar, cigarettes? What, if any, links will develop between the tobacco and pharmaceutical industry and what are the implications for TC?
International implications also need to be addressed. What impact do decisions made in one country have on the rest of the world, and what can be learnt from countries where both smoking rates and HR activities are low?
Philosophical
More broadly still, HR and NCPs raise questions about the fundamental purpose of public health. Is it an acceptable and effective public health practice to promote an addictive product? How does this vary between cultures and classes? What, if any, impact does HR have on the individual’s sense of agency and his or her ability to address wider health behaviours?
Finally, does the resulting accumulation of corporate power present any threats to TC or public health more generally?
Conclusion
There is a new sense of uncertainty in tobacco control. THR has been presenting alternate perspectives for some years in an appropriately cautious fashion, but the sudden arrival on the scene of heavily marketed e-cigarettes and other NCPs has greatly energised the debate. It is vital that the tobacco control movement agrees a unified strategy to address these developments; amidst all the uncertainty there is one certainty: any divisions will be ruthlessly exploited by vested interest. This taxonomy of harm reduction research provides a first step towards this unified strategy.