Here’s what is new in The BMJ today.
 • Practice
• Practice
Many GPs may find the challenge of assessing eyes and vision in infants and preschool children intimidating. Andrew Blaikie and Gordon Dutton offer advice—with multiple video demonstrations and tutorials.
 • Clinical review
• Clinical review
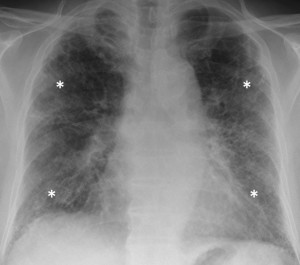
How confident are you in diagnosing interstitial lung diseases? How many of this heterogeneous set of conditions can you name? Radiologist and physician team Adam Wallis and Katherine Spinks discuss everything from disease assessment and investigation to treatment in their clinical review on the topic.
• Open data
You may have heard of the recent World Health Organization statement on clinical trial data transparency. But did you know the Nordic Trial Alliance came out with an even bolder declaration: “to make clinical research conducted in the Nordic region the most trusted clinical research in the world”? There is a growing understanding that data sharing is a fundamental quality of trustworthy research, and in this spirit, editors Elizabeth Loder and Trish Groves announce that The BMJ has extended its policy of requiring individual participant level data sharing to all trials, not just drugs and devices.
 • Conflicts of interest
• Conflicts of interest
Freedom of information laws allow citizens to know what their government is up to. But making use of those laws can be difficult. In a personal view, Benjamin J F Dean says the General Medical Council spent “more than £4000 on legal fees in resisting my freedom of information request.”
It’s not hard to see why: a supposedly “independent” review of UK postgraduate medical training chaired by David Greenaway—and criticized by the BMA, Royal College of Physicians, and trainee groups—made use of many “secret meetings with senior politicians and civil servants throughout the review.” One email, also obtained under freedom of information, sheds light on why the GMC thought the secrecy so necessary: “Disclosure would undoubtedly impact on future relationships with both these individuals and the departments they represent.” But Dean counters, and asks whether secrecy or transparency actually better serves patient safety.
• EMA “tightens” rules on revolving door with pharma industry
The European Medicines Agency (EMA) announced this week that it has updated its rules on declarations of interest of scientific committee members and experts consulted by the regulator. Individuals declaring they plan to take a job in the pharma industry will have their involvement in EMA matters restricted, according to the announcement.
Is this a progressive, cutting edge policy? What does this say about EMA decisions prior to this policy? “EMA considers that employment in a pharmaceutical company is incompatible with an involvement in Agency activities,” the EMA press release stated. But what about substantial financial relationships with pharma among those employed elsewhere? How many former and active advisors and decision makers have competing financial interests? Why are questions like these difficult to answer? Is anything stopping the EMA, Food and Drug Administration (FDA), and other regulators from making their own public, searchable database of internal and external experts and their financial competing interests, like ProPublica’s Dollars For Docs?
Peter Doshi, associate editor, The BMJ.
