Tremendous advances have been achieved in the therapeutic armament of disease modifying therapies (DMT) in relapsing-remitting multiple sclerosis (RRMS), leading to the concept of “no evidence of disease activity” (NEDA). This concept measures the success of treatment determined by the absence of clinical worsening (EDSS increase) and radiological activity (new/enlarging T2 lesions or gadolinium-enhancing lesions) in a control MRI study. However, despite patients continuing in NEDA during a stable DMT, studies have shown worsening of walking speed, cognition and brain atrophy. Therefore, further biomarkers are needed to monitoring subtle change during clinical follow-up.
In this issue of JNNP, Harel and colleagues explore microstructural brain white matter injury in patients with RRMS meeting NEDA criteria by using diffusion tensor imaging (DTI). They performed a retrospective study of RRMS patients who had undergone longitudinal DTI assessment. Patients were stratified into two groups according to NEDA criteria: (i) NEDA group and (ii) evidence of disease activity (EDA) group. The annual rate of DTI change was estimated based on the fractional anisotropy (FA) and mean diffusivity (MD) values. A total of 85 patients were included; from those, 39 were in the NEDA group. Of relevance, both groups showed longitudinal DTI changes, but the yearly FA decrease was higher in the EDA group than in the NEDA group, without statistically significant differences in MD values (figure 1). Interestingly, DTI variables correlated with disability and fatigue.
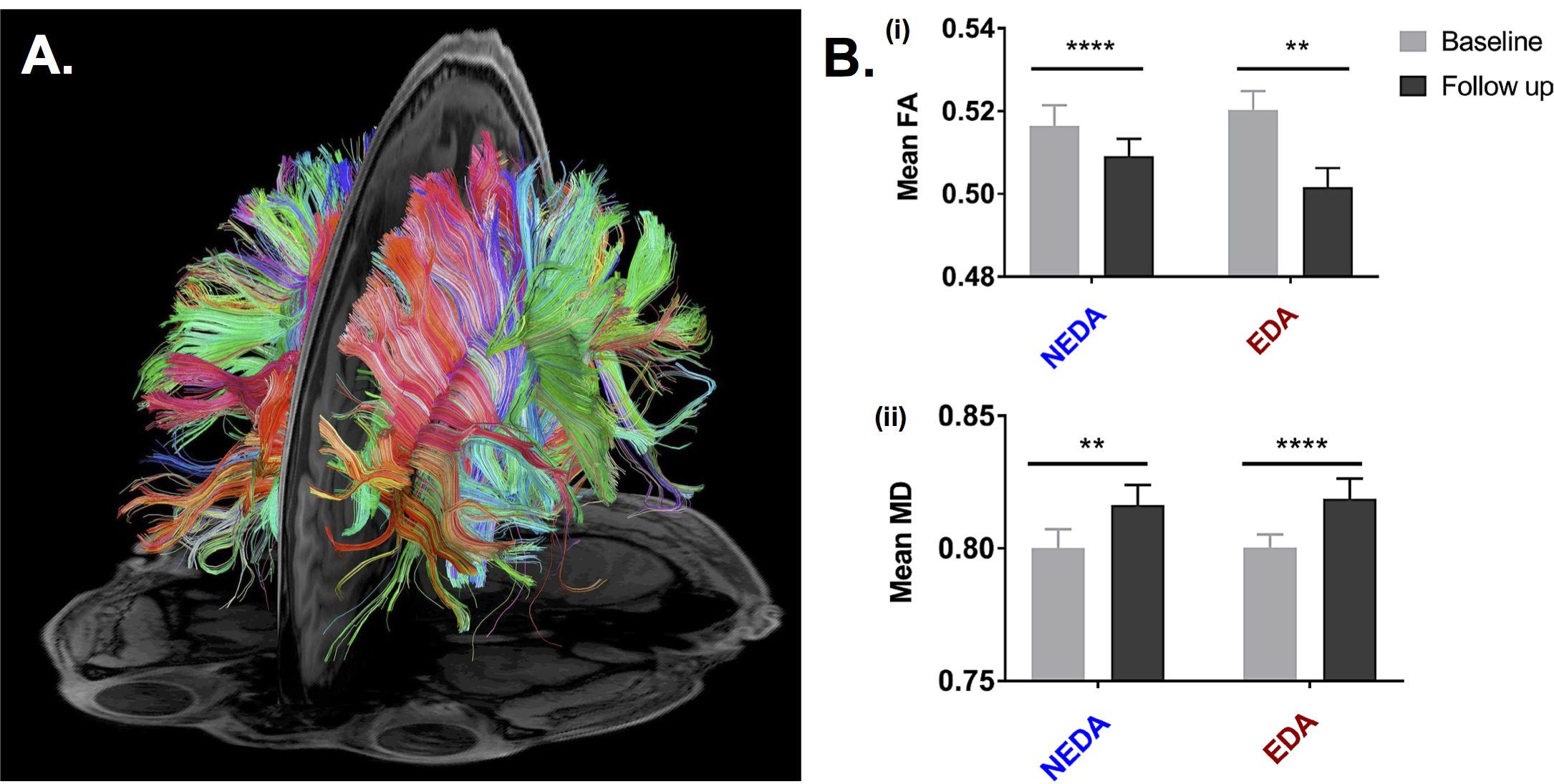
This study determines the existence of continuous white matter microstructural deterioration in patients with RRMS, independently of the NEDA criteria status. These changes were measured over a short-term follow-up period, suggesting fast dynamics of disease and of the potential neurodegenerative mechanism involved in this process, despite the absence of inflammatory lesions. These results also indicate the high sensitivity of DTI metrics in assessing white matter integrity in RRMS patients, providing proof of the limitation of conventional MRI studies for monitoring disease activity. From a clinical-radiological perspective, it has been demonstrated that demyelination and axonal degeneration results in DTI changes (decreased FA and increased MD), which correlate with disability and tissue injury. Overall, this finding argues in favor of the use of DTI as an additional MRI sequence for monitoring disease activity in RRMS patient. Further clinical trials will define which therapeutic strategies limit DTI worsening and its long-term consequences affecting disability and quality of life.
Read more at https://jnnp.bmj.com/content/89/9/977