‘the current system is failing, and awareness isn’t ‘variable’, it is wholly inappropriate and unethical.’
Carl Heneghan

The UK’s House of Commons Science and Technology Committee released its report on clinical trials transparency last week and it makes for a sorry read.
Clinical trials transparency means ensuring clinical trials are recorded in a publicly-accessible registry, summary results are published within a set time upon trial completion (usually 12 months) and results are reported in full.
UK and EU rules and guidelines aim to ensure these three pillars of transparency are upheld. Yet, about half of clinical trials are still unreported. The Sci-Tech Committee is not impressed:
‘many of the clinical trials taking place in the UK remain unregistered and unpublished and their data continue to be unavailable to both the general public and the scientific community. This is unacceptable and we have not been impressed by the Government’s efforts to resolve this important issue.’
Of the three pillars of transparency I thought trial registration was largely sorted. I was wrong.
‘In 2013, the Health Research Authority (HRA) made it a condition of a trial receiving a ‘favourable opinion’ from a research ethics committee that the trial must be registered—or a deferral for specific reasons requested—before participants are recruited.’
‘The HRA subsequently conducted several audits of registration, with follow-up contact with lead investigators where non-compliance was found. These demonstrated that registration was still not universal, even among trials that had received a favourable opinion, and that some who fell short on their compliance ignored contact from the HRA. The HRA’s 2017 audit revealed that 32% of 599 studies that received a ‘favourable opinion’ (and no agreed deferral) could not be found on a publicly accessible registry.
If I have this right, registration is a precondition of a favourable ethical approval; but this isn’t happening in a third of cases. The HRA concluded that “awareness that the requirement to register a clinical trial as a condition of favourable opinion was variable.‘
My conclusion, is the current system is failing, and awareness isn’t ‘variable’, it is wholly inappropriate and unethical.
Since 2014 the European Commision has required that all trials in the EU clinical trials register have to post results within 12 months. Here are today’s results from the eu.trialstracker.net/ – only 51.4% of trials sponsors have reported – I’d, yet again, call that failing.
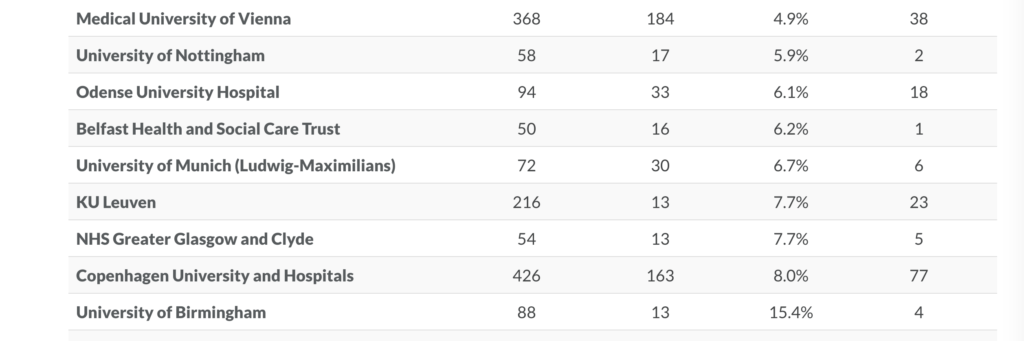
What’s also interesting is who is reporting results? If you order the EU Trials Tracker by percentage reported, the industry is at the top. If you look near the bottom, universities are largely failing in their legal requirements. In The UK, for example, the University of Nottingham complies with its requirements for only 17 of 58 trials (5.9%).
If you work at this university – or one of the others on the list – you may want to point these failings out. And if you work in the NHS or Public Health England you may also want to point out ‘It is particularly disappointing that trusted bodies such as Public Health England and a range of NHS Foundation Trusts are also failing to report results from clinical trials.’
Wherever you look trials transparency is failing. ‘Our predecessor Committee concluded in 2013 that it had “not been impressed” by the Government’s efforts to resolve the problem of un-registered, non-reported and mis-reported clinical trials. We believe that while there have been some improvements there is still much more to be done.’
THE Sci-tech Committee has therefore tasked the UK’s Health Research Authority to ‘introduce a system of sanctions to drive improvements in clinical trials transparency, such as withdrawing favourable ethical opinion or preventing further trials from taking place, and the Government should consult specifically on whether to provide the HRA with the statutory power to fine sponsors for non-compliance.’
The question is, therefore, by 2022 – when we’ll likely revisit these issues – will trials transparency still be failing?
References
- 10th Report – Research integrity: clinical trials transparency | PDF version 10th Report – Research integrity: clinical trials transparency (
 PDF )
PDF )
BMJ Evidence-Based Medicine – original evidence-based research, insights and opinion
Read more in the Welcome to BMJ Evidence-Based Medicine Editorial.
Competing interests
Carl has received expenses and fees for his media work including BBC Inside Health. He holds grant funding from the NIHR, the NIHR School of Primary Care Research, The NIHR Oxford BRC and the WHO. He has also received income from the publication of a series of toolkit books. CEBM jointly runs the EvidenceLive Conference with the BMJ and the Overdiagnosis Conference with some international partners which are based on a non-profit model. CH works with Ben Goldacre who leads the Trials Tracker’s in the EBM Datalab and is an advisor to the World Health Organization International Clinical Trials Registry Platform (ICTRP)