In a 1-year longitudinal study recently published in JNNP, ‘Neurochemical correlates of functional decline in amyotrophic lateral sclerosis’, ultra-high field 1H-MRS scans were used in 19 ALS patients by Cheong and colleagues to explore the relationship between neurochemical brain changes and functional decline. Specifically, the authors determined the regional levels of (i) N-acetylaspartate:myo-inositol (tNAA:mIns) from the upper limb motor cortex and (ii) glutamate+glutamine (Glx) from the pons and revealed changes in these neurochemical levels in patients with worsening upper limb and bulbar function, respectively. In contrast, patients with stable clinical function (as measured by ALSFRS-R) and healthy controls did not exhibit neurochemical changes in these regions over this time.
Although prior MRS studies in ALS have consistently supported reduced neuronal integrity in the motor region, this study expands previous analyses to suggest that regional neurochemical variability mirrors clinical variability within the ALS patient group, and is associated with the functional decline of the corresponding body region. Although the eventual longitudinal cohort in this analysis was small (with only 10 patients completing all 3 visits), such regional neurochemical changes may act as a key driver in understanding the basis of clinical disease heterogeneity. Another interesting aspect in this study was that of the Glx findings in the pons, which were comparatively normal in early stages of disease but showed a longitudinal increase in patients with worsening bulbar function. This strengthens the hypothesis of glutamate metabolism abnormalities in the pathophysiology of ALS, and indirectly supports glutamate excitotoxicity as a propagator of neurodegeneration. At what stage of the disease process these changes become evident, however, remains conflicting, and requires further investigation.
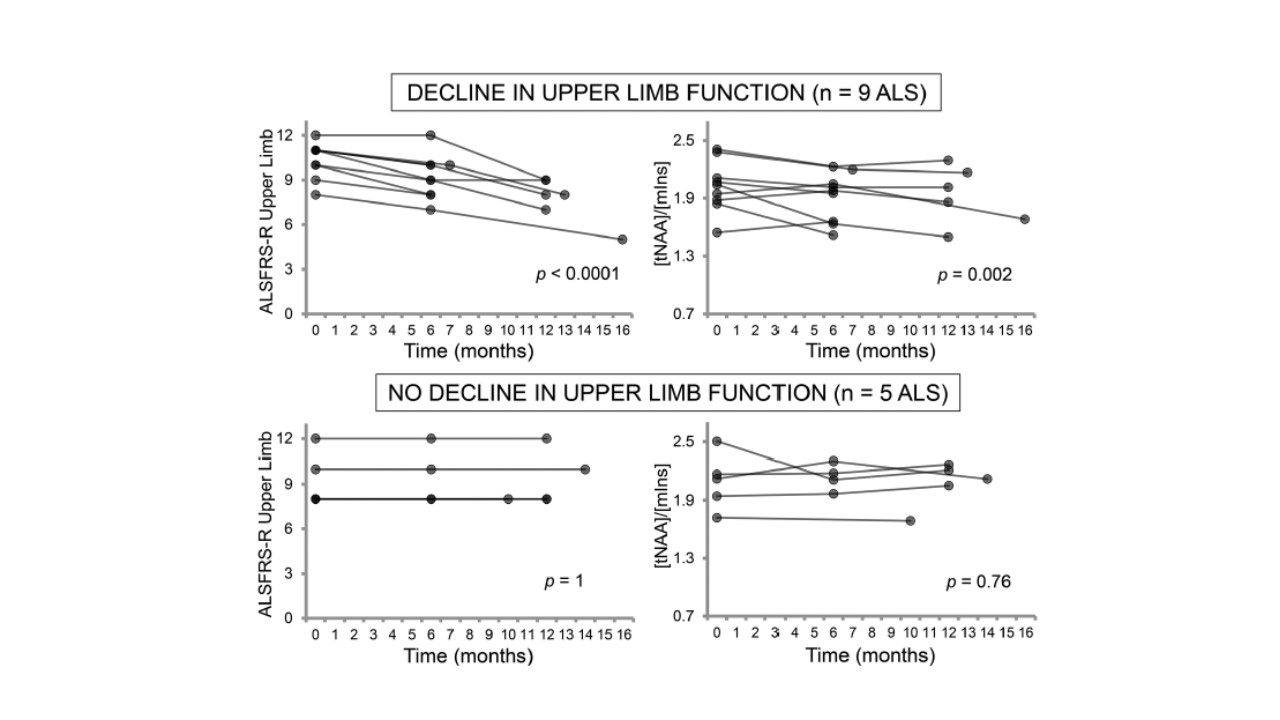
In the overall reflection of the correlations shown in this study, it is also important to consider that the ALSFRS-R is not a specific functional marker for upper (cortical) motor neuron involvement, and does not robustly discriminate between the degree of upper versus lower (peripheral) motor neuron involvement. Regardless, the patterns of cortical change presented by Cheong et al offer a unique regional insight into variability of the brain’s neurobiochemical milieu in disease and its association with ALS clinical features. Such pattern delineations are clearly essential to understand, as they may also underpin critical aspects of prognosis and survival. These findings will now need to be expanded to larger patient cohorts in longitudinal studies to further define mechanistic links, and to ultimately enable better understanding of disease processes in ALS.