Professor El-Omar has selected Professor Alexander C Ford from the Leeds Gastroenterology Institute/Leeds Institute of Medical Research, St. James’s University Hospital, Leeds, UK to do the next #GUTBlog. Professor Ford is the first author on this paper, with his co-authors being Heidi M Staudacher and Professor Nicholas J Talley in Australia.
The #GUTBlog focusses on the Recent Advances in Clinical Practice paper “Postprandial symptoms in disorders of gut-brain interaction and their potential as a treatment target” which was published in paper copy in GUT in July 2024.
Professor Ford writes:
“Postprandial, or meal-related symptoms, such as abdominal pain, early satiation, fullness, or bloating, are often reported by patients with disorders of gut-brain interaction (DGBI), including functional dyspepsia (FD) or irritable bowel syndrome (IBS). Approximately 60% of patients with FD and 25% of patients with IBS, respectively, report gastrointestinal symptoms that are associated with ingestion of a meal. The triggering of symptoms after eating in some patients with DGBI suggests that food intake is integral to symptom generation and propagation in in a subset of patients with these disorders.[1, 2] We, therefore, chose this topic as the subject of our Recent Advances in Clinical Practice article,[3] which was commissioned by Professor El-Omar, the editor-in-chief of Gut.
In this article, we propose that postprandial symptoms represent a distinct pathophysiological process in a subset of patients with DGBI. A physiological or psychological stressor leads to loss of tolerance to a previously tolerated oral food antigen, enabling interaction of both the microbiota and the food antigen itself with the immune system. This leads to a localised immunological response, with activation of eosinophils and mast cells, and the release of inflammatory mediators, including histamine and cytokines (Figure). The inflammatory mediators released may then have more widespread systemic effects, including triggering nociceptive nerve fibres and even altering mood.[4] This is supported by a mouse model of IBS, in which an acute enteric infection with C. rodentium led to an adaptive immune response to food antigens, via activated mast cells primed with allergen-specific IgE.[5] This response was limited to the gastrointestinal tract and the ultimate sequelae were abdominal pain, with abnormal pain signalling, and diarrhoea. These are the hallmarks of IBS.
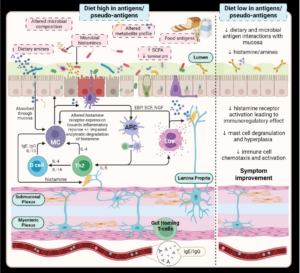
Although this is a controversial hypothesis, it fits within the concept of the bi-directional brain-gut axis,[6] which is proposed to be involved in DGBI. It would also explain why specific dietary changes or drugs are of particular benefit in some patients, something we reviewed in detail within the article.
A diet low in fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs), may have benefits in DGBI beyond the osmotic effects of FODMAP exclusion, and which relate to anti-inflammatory effects,[7] avoidance of activation of pain-related regions of the brain,[8] or elimination of potential food antigens or gluten. Similarly, gluten-free diets, food antigen elimination diets, or IgG-based food sensitivity diets may have beneficial effects via elimination of common food antigens, such as wheat, soy, egg, or milk, or IgG4 antibody-induced inflammation.[9-12] Finally, bioactive food chemicals, including salicylates, and biogenic amines, such as histamine, may induce postprandial symptoms. Hence, salicylate restriction or a low histamine diet will benefit some patients with IBS or FD.[13]
In terms of drugs, proton pump inhibitors reduce duodenal eosinophil counts in FD, and this reduction correlates with symptom improvement.[14, 15] Histamine-1 and histamine-2-receptor antagonists, as well as mast cell stabilisers, also have benefits in some patients with FD and IBS.[16-19] Finally, tricyclic and tetracyclic antidepressants, which are used as gut-brain neuromodulators in DGBI, have anti-histaminergic effects among their many actions. Tricyclic antidepressants are efficacious for IBS and FD in meta-analyses and large randomised trials.[20-23] There is also preliminary evidence that mirtazapine, a tetracyclic antidepressant, may be beneficial in patients with DGBI.[24][25]
Although it is unlikely that food antigens driving intestinal immune activation is the explanation for postprandial symptoms in all patients with FD and IBS, we believe that if this hypothesis is correct in even a subset, it is paradigm-shifting, offering new and more effective treatment possibilities for patients with these difficult to manage symptoms.”
REFERENCES
1 Carbone F, Vanuytsel T, Tack J. Analysis of postprandial symptom patterns in subgroups of patients with Rome III or Rome IV functional dyspepsia. Clin Gastroenterol Hepatol 2020;18:838-46.
2 Arsie E, Coletta M, Cesana BM, Basilisco G. Symptom-association probability between meal ingestion and abdominal pain in patients with irritable bowel syndrome. Does somatization play a role? Neurogastroenterol Motil 2015;11:12510.
3 Ford AC, Staudacher HM, Talley NJ. Postprandial symptoms in disorders of gut-brain interaction and their potential as a treatment target. Gut 2024;73:1199-211.
4 Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007;132:913-20.
5 Aguilera-Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, Appeltans I, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021;590:151-6.
6 Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 2012;61:1284-90.
7 Zhou SY, Gillilland M, 3rd, Wu X, Leelasinjaroen P, Zhang G, Zhou H, et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest 2018;128:267-80.
8 Wu J, Masuy I, Biesiekierski JR, Fitzke HE, Parikh C, Schofield L, et al. Gut-brain axis dysfunction underlies FODMAP-induced symptom generation in irritable bowel syndrome. Aliment Pharmacol Ther 2022;55:670-82.
9 Fritscher-Ravens A, Pflaum T, Mosinger M, Ruchay Z, Rocken C, Milla PJ, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019;157:109-18.e5.
10 Fritscher-Ravens A, Schuppan D, Ellrichmann M, Schoch S, Rocken C, Brasch J, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012-20.e4.
11 Arabpour E, Alijanzadeh D, Sadeghi A, Khoshdel S, Hekmatdoost A, Kord-Varkaneh H, et al. Gluten restriction in irritable bowel syndrome, yes or no?: A GRADE-assessed systematic review and meta-analysis. Front Nutr 2023;10:1273629.
12 Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomised controlled trial. Gut 2004;53:1459-64.
13 Tuck CJ, Malakar S, Barrett JS, Muir JG, Gibson PR. Naturally-occurring dietary salicylates in the genesis of functional gastrointestinal symptoms in patients with irritable bowel syndrome: Pilot study. JGH Open 2021;5:871-8.
14 Wauters L, Ceulemans M, Frings D, Lambaerts M, Accarie A, Toth J, et al. Proton pump inhibitors reduce duodenal eosinophilia, mast cells, and permeability in patients with functional dyspepsia. Gastroenterology 2021;160:1521-31.e9.
15 Potter MDE, Wood NK, Walker MM, Jones MP, Talley NJ. Proton pump inhibitors and suppression of duodenal eosinophilia in functional dyspepsia. Gut 2019;68:1339-40.
16 Potter MDE, Goodsall TM, Walker MM, Talley NJ. Dual histamine blockade for the treatment of adult functional dyspepsia: A single centre experience. Gut 2020;69:966.
17 Decraecker L, De Looze D, Hirsch D, De Schepper H, Arts J, Caenepeel P, et al. Treatment of non-constipated irritable bowel syndrome with the histamine 1 receptor antagonist ebastine: A double-blinded, randomized, placebo-controlled trial. Gut 2024;73:459-69.
18 Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010;59:1213-21.
19 Lobo B, Ramos L, Martínez C, Guilarte M, González-Castro AM, Alonso-Cotoner C, et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United European Gastroenterol J 2017;5:887-97.
20 Ford AC, Lacy BE, Harris LA, Quigley EM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol 2019;114:21-39.
21 Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: Systematic review and meta-analysis. Gut 2017;66:411-20.
22 Ford AC, Wright-Hughes A, Alderson SL, Ow PL, Ridd MJ, Foy R, et al. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment in primary care (ATLANTIS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;402:1773-85.
23 Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multi-center, randomized, controlled study. Gastroenterology 2015;149:340-9.
24 Tack J, Ly HG, Carbone F, Vanheel H, Vanuytsel T, Holvoet L, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol 2016;14:385-92.
25 Khalilian A, Ahmadimoghaddam D, Saki S, Mohammadi Y, Mehrpooya M. A randomized, double-blind, placebo-controlled study to assess efficacy of mirtazapine for the treatment of diarrhea predominant irritable bowel syndrome. BioPsychoSocial medicine 2021;15:3.
SOCIAL MEDIA
@alex_ford12399