Professor El-Omar has chosen Professor Paul O’Toole to do the #GUTBlog this month. Professor O’Toole is a Professor of Microbial Genomics, Head of School of Microbiology and Principal Investigator in APC Microbiome Ireland, an SFI funded centre at University College Cork, Ireland. He leads a team in the Extremes of Life programme, examining the role of the gut microbiome in healthy aging.
This #GUTBlog focusses on the paper ‘Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries’ published in paper copy in Gut in July 2020.

“The background to this study was our long-standing interest in the gut microbiome of older people, investigating if and how it might impact on health. The gut microbiome is a community of hundreds of species whose properties and metabolic products have been linked to altered risk of a range of non-communicable diseases including Inflammatory Bowel Disease, Irritable Bowel Syndrome, Type 2 diabetes, cardiovascular disease and obesity. It is not clear to what extent these microbiome changes are causative or consequential, but what is indisputable is that gut bacteria are solely responsible for liberating metabolizable compounds from fibre and complex polysaccharide in the diet. Fermentation in the large bowel releases short chain fatty acids and organic acids which act as an energy source for enterocytes, are anti-proliferative, and promote gut barrier function.
Our previous studies had established that older people with a low diversity diet had a lower diversity microbiome, and there were associations between loss of various microbes and frailty, inflammation and lower cognitive function (Claesson et al. 2012, Jeffery et al. 2016). However, we did not know if the lower health status was caused by the altered microbiome. Last year, we performed a 6 month dietary supplementation with 5 prebiotics (Tran et al. 2019), but the microbiome changes were modest and the impact on health parameters was a non-significant trend towards improvement. In an earlier Gut paper, we collaborated with a group in Naples to show that consumption of a Mediterranean diet (MedDiet) was associated with a higher diversity microbiome (De Filippis et al. 2015). In the study reported in Gut in July 2020 (Ghosh et al. 2020), we availed of an EU funded project called NuAge, anchored by Professor Claudio Franceschi in the University of Bologna, to test the effect of a MedDiet on the microbiome and health of 600 people across 5 European countries including the UK.

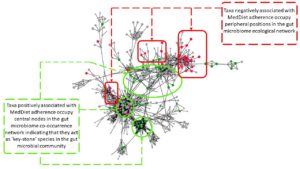
A significant element of this study was that we performed a remodelling of the whole diet, not just a supplementation, and we enrolled the subjects for one year. The subjects were all over age 65, and pre-frail or non-frail, but were expected to show at least some signs of frailty development over the course of one year. We found that the MedDiet group, but not the control group who continued on their own diet, showed a significant reduction in onset of frailty, inflammation and loss of cognitive function (Ghosh et al. 2020). This effect was linked with the increased relative abundance of a group of microbes, many of whom had been linked to healthier status in previous studies, and depleted abundance of microbes associated with disease. We constructed ecosystem models of the microbiome in the subjects receiving the MedDiet, which basically means mapping the networks into which the key microbiome members assemble (Figure 1). We found that the responsive bacterial taxa occupied key positions in the network, at nodes one would predict would be hard to change unless with a long-term radical change in diet. This may explain why short-term diet changes have short-term effects.
We are really keen to understand how particular gut bacteria might promote healthier or unhealthy aging. We recently performed a metanalysis of the gut metagenome of 2,500 controls or diseases – IBD, colorectal cancer, cirrhosis, and type 2 diabetes, trying to separate microbiome disease signatures from aging microbiome signatures (Ghosh, Das et al. 2020). The results largely corroborate those we found experimentally in the Gut paper, and provide a short-list of bacteria that appear to contribute to delayed onset of frailty. We used computer modelling to predict what the relevant metabolites might be, but next we will experimentally validate that. If we cannot get older people to consume a MedDiet, and there are plenty of clinical or sociological reasons why that might be challenging, maybe we can provide the “missing microbes” to restore biochemical function to the gut metagenome. We also theorize about how the gut microbiome might affect innate or adaptive immunity; having spent the last 13 years trying to improve the health of older people, we are struck by how chillingly brutal the current pandemic is in its toll upon this stratum of our society. Nothing trumps infection control. However, in the same way as the gut microbiome modulates response to checkpoint inhibitors used to treat certain cancers, we plan to investigate if it might impact on COVID-19 susceptibility, disease progression, or response to future vaccines in older subjects.”
References
Claesson, M. J., I. B. Jeffery, S. Conde, S. E. Power, E. M. O’Connor, S. Cusack, H. M. Harris, M. Coakley, B. Lakshminarayanan, O. O’Sullivan, G. F. Fitzgerald, J. Deane, M. O’Connor, N. Harnedy, K. O’Connor, D. O’Mahony, D. van Sinderen, M. Wallace, L. Brennan, C. Stanton, J. R. Marchesi, A. P. Fitzgerald, F. Shanahan, C. Hill, R. P. Ross and P. W. O’Toole (2012). “Gut microbiota composition correlates with diet and health in the elderly.” Nature488: 178-185.
Ghosh, T. S., M. Das, I. B. Jeffery and P. W. O’Toole (2020). “Adjusting for age improves identification of gut microbiome alterations in multiple diseases.” Elife9.
Jeffery, I. B., D. B. Lynch and P. W. O’Toole (2016). “Composition and temporal stability of the gut microbiota in older persons.” ISME J.10(1): 170-182.
Tran, T. T. T., F. J. Cousin, D. B. Lynch, R. Menon, J. Brulc, J. R. Brown, E. O’Herlihy, L. F. Butto, K. Power, I. B. Jeffery, E. M. O’Connor and P. W. O’Toole (2019). “Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity.” Microbiome7(1): 39.