Gender dysphoria occurs when a person experiences discomfort or distress because of a mismatch between their biological sex and gender identity. Gender dysphoria can arise in childhood and adolescent which raises many questions about how best to handle the condition. This post sets out some of the current evidence for gender-affirming hormones in adolescents and children to aid decision making.
How big a problem is gender dysphoria?
Prevalence estimates suggest male-to-female cases outnumber female-to-male cases, with 1 per 10,000 males and 1 per 27,000 females affected by gender dysphoria, although estimates vary depending on the setting. These rates would qualify for orphan designation status (defined by the European Union as less than 5 in 10,000 of the general population).
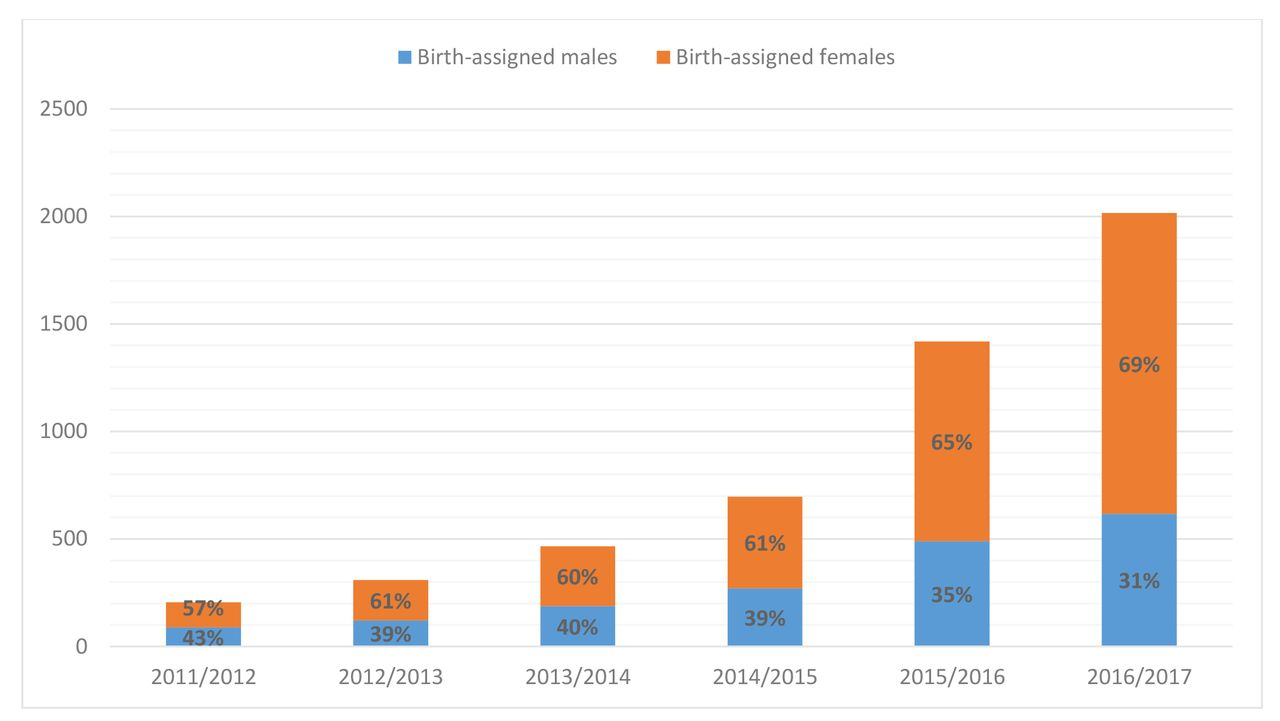
We know higher rates are observed in Western Europe and America, but the exact prevalence is difficult to estimate because the number of children and adolescents referred to services is still rising. As an example, UK referrals to the national Gender Identity Development Service (GIDs) has risen exponentially since 2011.
Reference: Referrals to UK GID services: Assessment and support of children and adolescents with gender dysphoria. Arch Dis Child 2018;103:631–6. doi:10.1136/archdischild-2018-314992
Treatments options for Gender Dysphoria
The World Professional Association for Transgender Health (WPATH) Guidelines, on the clinical care of transgender adolescent, set out three stages of gender-affirming interventions with progressive levels of irreversibility:
- Stage 1, puberty suppression
- Stage 2, gender-affirming hormones
- Stage 3, gender-affirming surgery
Guidelines require puberty to have begun (Tanner stage 2, when pubic hair and breast buds appear) before any intervention is agreed. This is because gender dysphoria may resolve once puberty begins. In 2008 the Endocrine Society approved puberty blockers for transgender adolescents as young as 12 years old.
To find the evidence for treatment options we first searched for systematic reviews. We used PubMed Clinical Queries to search for the reviews (see here). We found two up to date reviews with overlapping trial results:
- Hormonal Treatment in Young People With Gender Dysphoria: A Systematic Review. Chew D, Anderson J, Williams K, May T, Pang K. Pediatrics. 2018 Apr;141(4). doi: 10.1542/peds.2017-37422.
- Gender-affirming hormones and surgery in transgender children and adolescents. Mahfouda S, Moore JK, Siafarikas A, Hewitt T, Ganti U, Lin A, Zepf FD. Lancet Diabetes Endocrinol. 2018 Dec 6. doi: 10.1016/S2213-8587(18)30305-X.
There are other reviews you might want to take a look at, such as The effect of cross-sex hormonal treatment on gender dysphoria individuals’ mental health: a systematic review, and a Systematic Review of the Effects of Hormone Therapy on Psychological Functioning and Quality of Life in Transgender Individuals.
We focused on the latest reviews in children and adolescents that reported a range of clinical outcome to inform decision making. The first review Hormonal Treatment in Young People With Gender Dysphoria [1], searched Medline, Embase, and PubMed to June 10, 2017, and assessed risk of bias using a modified version of the Quality in Prognosis Studies, and published a protocol registered at PROSPERO (CRD42017056670). The second, Gender-affirming hormones and surgery in transgender children and adolescents [2], searched MedLine and Embase, included studies when the mean or median age of the sample was below 18 years, reported information on the limitations of each study and set out research recommendations.
Together these reviews included 16 studies with 1,132 participants (transgender males (54%); transgender females (37%) and (7.6%) control subjects reported. Controls were not matched for important confounders, which means caution should be applied to any conclusions drawn. We found no randomized controlled trials or controlled trials.
Stage 1, Puberty suppression treatments
Gonadotrophin-releasing hormone agonists (GnRHa) acts on GnRH receptors to suppress gonadotropin release. In females GnRHa reduces the secretion of LH and FSH; in males, it shuts down gonadal testosterone production. For this reason, they are often referred to as puberty blockers. Little is known about the safety profile in the context of gender dysphoria, particularly the long-term effects, and use is based largely on the effects of treatment of central precocious puberty.
The clinically used GnRH agonists are available in the following formulations:
- Short-acting injection: buserelin, histrelin, leuprorelin, triptorelin
- A long-acting depot injection or injected pellet: leuprorelin, triptorelin
- Injected implant: buserelin, goserelin, leuprorelin
- Surgically implanted pellet: histrelin, leuprorelin
- Nasal spray: buserelin, nafarelin
Some evidence suggests that children will change their minds as they age: just under three-quarters of pre-pubescent children attending gender identity clinics may not want to change their gender once puberty starts: a prospective study of 77 gender dysphoric children (59 boys, 18 girls; mean age 8.4 years, range 5–12 years) referred to one clinic found that after 3.4 years of follow-up 27% remained gender dysphoric.
Ten studies analysed the effects of puberty blockers: the median age of starting in transgender males in these trials was 15.0 years (median range 13.5 to 15.8 years), and in females, 15.1 years (range 13.6 to 16.5 years).
Vlot 2017 reported the lowest median age in boys of 13.5 years; Schagen 2016, funded by an unrestricted grant from Ferring the makers of the study drug triptorelin, reported a median age of 13.6 years in transgender females for starting treatment. Six studies were funded by industry: 4 received funding from Ferring (Delemarre-van de Waal 2006, Staphorsius (2015), Schagen 2016 and Hannema 2017).
The numbers in the ten studies are small and most are retrospective case reports or small case series. Many are done in single clinics and lack long term longitudinal outcomes on the effects (both benefits and harms) of puberty blockers. It is also hard to disentangle effects from the use of gender affirming hormones. We found four studies reporting on the use of GnHRa alone: Schagen 2016; Staphorsius 2015; Costa 2015and Delemarre-van de Waal 2006.
Schagen 2016 studied the effects of Triptorelin in gender dysphoric adolescents and reported that ‘treatment did not have to be adjusted because of insufficient suppression in any subject.’ They concluded further studies should evaluate whether the effects on height and body composition can be reversed during subsequent GAH treatment. Costa 2015 reported that global functioning after psychological support and puberty suppression was improved. Delemarre-van de Waal 2006 reported GnRHa treatment appeared to be important for the management of gender identity in transsexual adolescents. Finally, Staphorsius 2015, determined whether the performance on the Tower of London task cognitive task was altered with GnRHa and found no significant effects on task scores.
Problems within these studies, however, make it difficult to assess whether early pubertal changes regress under GnRHa treatment and whether prolonged puberty suppression is safe. For example, there is a lack of controls, and in one study that included controls, these were inadequate as relatives and friends of the participants were asked to participate, serving as age-matched controls. A lack of blinding was also problematic. One study (Costa 2015) that focused on a measure of psychosocial well-being highlighted that getting older has previously been positively associated with maturity and well-being (see Getting older, getting better? Personal strivings and psychological maturity across the life span.)
Stage 2, Gender-affirming cross-sex hormone hormones (CSHs)
Oestrogens and testosterone induce masculine or feminine physical characteristics, and should only be taken in the context of medical supervision to monitor risks (e.g., polycythaemia in transgender males, venous thromboembolism in transgender females).
For transgender females, oestrogen therapy alone is often insufficient to produce the desired feminising effects. Other treatments are therefore used in an off label manner. For example spironolactone, an aldosterone antagonist with weak oestrogenic properties is commonly used to support oestrogen therapy – off label. Cyproterone acetate has progestational and antiandrogenic properties, but it can lead to hepatic toxicity including jaundice, hepatitis. Hepatic failure has also been reported (fatalities reported, usually after several months, at dosages of 100 mg and above).
Specific effects of gender affirming hormones
Psychological effects
Young transgender people may have mental health problems, including anxiety, and suicidal ideation. De Vries 2014 (n =55) assessed gender dysphoria, body satisfaction, at baseline, puberty suppression, and in adulthood. De Vries 2011 reported on the original cohort (n=70) that showed that emotional problems and depressive symptoms decreased, while general functioning improved significantly during puberty suppression. High levels of bias with study participation mean the results should be treated with caution. The study found a decrease in gender dysphoria after surgery. However, it was not possible to disentangle the psychological benefits of hormone treatments from surgical interventions.
Cognitive and brain-related effects
Neuroimaging studies suggest CSHs affect brain structure and circuitries, ventricular volume and thickness, hypothalamic neuroplasticity, and functional connectivity. One study, Burke (2016) (n=62) investigated GAHs and brain function in adolescents, and reported that testosterone therapy in transgender males (n=21 mean age 16.1) was associated with altered cognitive processes, as assessed by the mental rotation task (MRT), a measure of visuospatial working memory that elicits cognitive sex differences. The study concluded that transgender males have atypical sexual differentiation of brain areas involved in visuospatial cognitive functioning.
Bone development
Klink 2015 found that lumbar spine bone mineral density scores fell during puberty suppression with GnRHa for transgender adolescent females but did not increase following oestrogen treatment. Endocrine Society Guidelines state monitoring BMD parameters in transgender adolescents is recommended both prior to and during gender-affirming hormonal treatment.
Haematological variables
Testosterone therapies stimulate erythropoiesis, and increases in haemoglobin and haematocrit are an anticipated physiological response. Jarin 2017 (n =116) reported that testosterone therapy in transgender males was associated with significant elevations in mean haemoglobin and haematocrit. Tack 2016 reported haemoglobin and haematocrit concentration variables increased but stabilised at six months. In transgender adolescent females estradiol. Olson-Kennedy 2018 report a significant decline in Hb concentrations after a 2-year course of estradiol.
Cardiovascular Health
Tack 2016; Jarin 2017 report no changes in LDL or triglycerides in the short term for transgender adolescent males. Olson-Kennedy 2018 report significant increases in triglyceride concentrations and HDL after two years of oestrogen treatment. None of the studies showed significant changes in mean total cholesterol concentrations. Olson-Kennedy 2018 report elevations in systolic and diastolic blood pressure with testosterone treatment after two years. Jarin 2017 reports no change in BP at six months. Jarin 2017, Olson-Kennedy 2018 and Tack 2016 report no changes in HbA, glucose, or insulin.
Conclusions
There are significant problems with how the evidence for Gender-affirming cross-sex hormone has been collected and analysed that prevents definitive conclusions to be drawn. Similar to puberty blockers, the evidence is limited by small sample sizes; retrospective methods, and loss of considerable numbers of patients in the follow-up period. The majority of studies also lack a control group (only two studies used controls). Interventions have heterogeneous treatment regimes complicating comparisons between studies. Also, adherence to the interventions is either not reported or inconsistent. Subjective outcomes, which are highly prevalent in the studies, are also prone to bias due to lack of blinding.
An Archive of Diseases in Childhood letter referred to GnRHa treatment as a momentous step in the dark. It set out three main concerns: 1) young people are left in a state of ‘developmental limbo’ without secondary sexual characteristics that might consolidate gender identity; 2) use is likely to threaten the maturation of the adolescent mind, and 3) puberty blockers are being used in the context of profound scientific ignorance.
The development of these interventions should, therefore, occur in the context of research, and treatments for under 18 gender dysphoric children and adolescents remain largely experimental. There are a large number of unanswered questions that include the age at start, reversibility; adverse events, long term effects on mental health, quality of life, bone mineral density, osteoporosis in later life and cognition. We wonder whether off label use is appropriate and justified for drugs such as spironolactone which can cause substantial harms and even death. We are also ignorant of the long-term safety profiles of the different GAH regimens. The current evidence base does not support informed decision making and safe practice in children.
Carl Heneghan
Editor in Chief BMJ EBM, Professor of EBM, University of Oxford
Tom Jefferson
Senior Associate Tutor University of Oxford
Visiting Professor Institute of Health & Society, Faculty of Medicine, Newcastle University
This post was updated on the 30th March, the 13th of April and the conflicts of Interest disclosures were added in full on the 21st May 2019. The full references to the systematic review articles were added as there was an error in the link to one of the reviews. The statement on Schagen 2016 was corrected to ‘treatment did not have to be adjusted because of insufficient suppression in any subject.’
[1] Hormonal Treatment in Young People With Gender Dysphoria: A Systematic Review. Chew D, Anderson J, Williams K, May T, Pang K. Pediatrics. 2018 Apr;141(4). doi: 10.1542/peds.2017-3742
[2] Gender-affirming hormones and surgery in transgender children and adolescents. Mahfouda S, Moore JK, Siafarikas A, Hewitt T, Ganti U, Lin A, Zepf FD. Lancet Diabetes Endocrinol. 2018 Dec 6. doi: 10.1016/S2213-8587(18)30305-X.
Competing interests
This evidence review was performed as part of a BBC Panorama documentary: Trans Kids: Why Medicine Matters, release date: 27 February 2019.
Carl has received expenses and fees for his media work (including payments from BBC Radio 4 Inside Health). He has received expenses from the WHO, FDA, and holds grant funding from the NIHR, the NIHR School of Primary Care Research, The NIHR BRC Oxford and previously the WHO. He has received financial remuneration from an asbestos case and given free legal advice on mesh cases. He has also received income from the publication of a series of toolkit books published by Blackwells. On occasion, he receives expenses for teaching EBM and is also paid for his GP work in NHS out of hours (contract with Oxford Health NHS Foundation Trust). He is Director of CEBM, which jointly runs the EvidenceLive Conference with the BMJ and the Overdiagnosis Conference with international partners, based on a non-profit making model. He is Editor in Chief of BMJ Evidence-Based Medicine and is an NIHR Senior Investigator. Full disclosure here. TJ received a fee from the BBC for this work. TJ was a co-recipient of a UK National Institute for Health Research grant (HTA – 10/80/01 Update and amalgamation of two Cochrane reviews: neuraminidase inhibitors for preventing and treating influenza in healthy adults and children; https://www.journalslibrary.nihr.ac.uk/programmes/hta/108001/). TJ was also in receipt of a Cochrane Methods Innovations Fund grant to develop guidance on the use of regulatory data in Cochrane reviews. TJ is occasionally interviewed by market research companies about phase I or II pharmaceutical products. In 2011–2014, TJ acted as an expert witness in a litigation case related to the antiviral oseltamivir, in two litigation cases on potential vaccine-related damage and in a labour case on influenza vaccines in healthcare workers in Canada. He has acted as a consultant for Roche (1997–1999), GSK (2001–2002), Sanofi-Synthelabo (2003) and IMS Health (2013). In 2014–2016, TJ was a member of three advisory boards for Boehringer Ingelheim. TJ was a member of an independent data monitoring committee for a Sanofi Pasteur clinical trial on an influenza vaccine, and has a potential financial conflict of interest on the drug oseltamivir. TJ is co-holder of a Laura and John Arnold Foundation grant for the development of a RIAT support centre (2017-2020) and Jean Monnet Network Grant, 2017-2020 for The Jean Monnet Health Law and Policy Network. TJ is an unpaid collaborator to the project Beyond Transparency in Pharmaceutical Research and Regulation led by Dalhousie University and funded by the Canadian Institutes of Health Research (2018-2022). Full disclosure here.
DISCLAIMER
The views and opinions expressed on this site are solely those of the original authors. They do not necessarily represent the views of the BMJ and should not be used to replace medical advice. All information on this blog is for general information, is not peer-reviewed, requires checking with original sources and should not be used to make any decisions about healthcare. No responsibility for its accuracy and correctness is assumed by us, and we disclaim all liability and responsibility arising from any reliance placed on such commentary or content by any user or visitor to the Website, or by anyone who may be informed of any of its content. Any reliance you place on the material posted on this site is therefore strictly at your own risk.