Culture of the gonococcus – some historical details
The 43 year period between two BJVD articles1, 2 incubated improvements in the diagnosis of gonorrhoea by laboratory culture. The following 47 gave birth to alternative tests (NAATs), more simple to administer, but whose automation brought loss of personnel and possibly skills: perhaps in microscopy, perhaps in laboratory culture.
In 1927 Colonel Harrison wrote1: “There are differences of opinion as to the value of cultures in the diagnosis of gonorrhoea. Personally I think them indispensable in the case of women and often valuable in male urethritis” (my emphasis).
Laboratory culture of Neisseria gonorrhoeae has always lacked 100% sensitivity. Sampling from multiple sites, on multiple occasions, was necessary to diagnose, to exclude, and, importantly, to monitor any advances, or fluctuations, in the efficiency of laboratory culture. The use of repeated tests to analyse the sensitivity of culture is now impossible, with the universal adoption of epidemiological treatment (before/without diagnosis).
Duncan Catterall’s 1970 article2 highlights sharp contrasts with today’s practice and prevalence [my comments follow his quotes]:
– “A full physical examination was performed on all the patients… They were all observed for at least 3 months.”
Measurement of BP, breast examination, abdominal palpation and urinalysis were standard for all new, and rebooked patients after one year. We were allocated more time with individual patients, almost all of whom were prepared to return for repeat tests.
– “gonococci were found in 95 (31.6%) of the women”
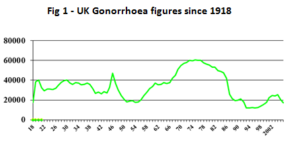
Gonorrhoea prevalence was higher then than nowadays; the mode of UK gonorrhoea incidence occurred in the mid-1970s (fig 1). Catterall’s was a consecutive series of 300 women who had attended, or been referred, because of vaginal discharge. A similar series today would be lucky to include 9, let alone 95, cases of gonorrhoea.
– “…even with a first-class cultural service, repeated examinations are needed to establish the diagnosis of gonorrhoea in women”
In the 1970s at least three sets of tests were taken on separate occasions to diagnose/exclude gonorrhoea in women. Even with multiple sets of tests, the culture results varied significantly between Centres.
The decade witnessed attempts at improving cultural sensitivity: a variety of selective but non-inhibitory mediums, often modifications of Thayer-Martin’s version, gradually replaced non-selective ones. Catterall used McLeod’s culture medium, with no antibiotics or antifungal agents, and diagnosed 60% of gonorrhoea at the first visit.
A series of publications from London clinics3 demonstrated laboratory culture’s lack of sensitivity but its gradual improvement. Results from the first set of tests at various Centres included 66% (Middlesex, 19702), 90% (St Thomas’, 19714), 91% (Middlesex, 19765), 88% (Charing X, 19766), 97% (St Thomas’, 19767), 98% (St Thomas’, 19788), 95% (Barts, 19799). All of these figures came from units with a particular interest in gonorrhoea and its diagnosis, but variation persisted.
– “Every patient had at least 4 pelvic examinations and the majority had 6 or more genital tests.”
Exclusion was as important as diagnosis. We knew that the first set of tests would miss some cases but we also knew that a number of gonorrhoea contacts would not have the disease, between 8% and 35% 10 (polarising views for and against epidemiological treatment). The same may be true today: Turner writes11 “Presumptive or epidemiological treatment of chlamydia and/or gonorrhoea accounts for a large number of suboptimal and unnecessary antibiotic prescriptions”, calculating (“a conservative assumption”) the probability of partner infection as 0.4.
– “gonococci were found [by microscopy] in only 67 (69%) of 95 consecutive cases”
This last quotation demonstrates how ‘a poor culture service flatters the microscopist12’: Suppose 50 of 100 genuine cases of gonorrhoea (50%) are identified by microscopy. If culture misses 20, microscopy will appear more sensitive, at 50 of 80 (62.5%). The same calculation applies to assessment of NAATs.
Microscopy in women at St Thomas’s found 50% compared with Catterall’s 69%, and a later retrospective survey13, from Catterall’s unit, noted the poorer sensitivity of culture in small clinics outside London. Again variation.
Use of Ian Phillips’ VCNT4 combined with scrupulous attention to detail, had improved culture sensitivity to 98% by 1978 (continuing at 97% in 198814).
Which brings me to the big question: how does the standard and variability of gonococcal culture today compare with (the best of) the 1970s? And does it matter?
In 1977 Morton15 wrote “The gonococcus is the most fastidious of organisms. It has long taxed the skill and ingenuity of bacteriologists.” The WHO, with Unemo and Ison’s imprimatur (2013)16, updates the caution: “Strict sample collection, transportation, and storage are crucial to maintaining viability”, listing vital criteria: “Number of sampling sites, technique and swabs used for collection of specimens; conditions and duration of transportation; composition and quality of the culture medium; inoculation and incubation conditions; and reagents and techniques used for the species identification of N. gonorrhoeae.”
In the 1970s, as now, we had Reference Laboratories, guidelines, and exhortations to quality control and yet there was still a measurable variation in outcomes of gonococcal culture between and within London’s ‘Centres of Excellence’.
Before I am overwhelmed by howls of protest from microbiologists (and epidemiologists), I wonder how many laboratories, whose ‘culture’ results provide today’s comparators for epidemiological and NAAT evaluations, look, have looked, at the performance of their transport/culture systems17, 18, in a similarly fastidious way?
What proportion of clinics or testing sites these days have all the index samples plated direct in clinic on to an appropriate selective but non-inhibitory medium (made up in-house as required, not commercial18), and placed directly in the clinic’s CO2/humidity/temperature-controlled incubator, with samples transferred to the on-site laboratory (dedicated gonorrhoea bench, dedicated gonorrhoea technician) twice daily, and with daily quality control to include correlation of microscopy of samples from the male urethra with their culture results.
This crucial monitoring of men’s samples enabled us to pick up problems with the incubators (clinic and laboratory), growth medium, a new technician on the ‘GC’ bench, or even sloppy plating-out in the clinic. If a discrepancy arose, we were aware of it in as few as 24 hours and were able to adjust our management of women, for whom culture was (and should still be) of so much greater importance.
While matching the earlier paragraph’s stringent conditions, culture in 1988 at St Thomas’ still missed 3% of cases of gonorrhoea in women at the first attempt14.
Nobody would disagree with the conclusion of a recent STI article19 that: “…the use of dual NAATs in the context of a population with low prevalence of gonorrhoea is likely to result in false positive results”, but one should also question any who assume that all the NAAT positive/culture negative results in their series are false positives.
I end with two cautions; one old, one new:
Taylor and Phillips (1980): “We conclude that although these transport-culture media performed well if incubated immediately and examined immediately, performance was not nearly so good under conditions that mimic more closely those actually obtaining in most clinics.” 18
And my own (2017): ‘a poor culture service flatters the NAAT, and may distort measures of prevalence and incidence’.
by Dr David Barlow, Emeritus Consultant Physician
References
- Harrison LW (Colonel) (1927) Gonorrhoea. Br.J.Vener.Dis. 3, 24-32
- Catterall RD (1970) Diagnosis of vaginal discharge. Br.J.Vener.Dis. 46, 122-4
- Barlow D (2007). R.D. Morton Memorial Lecture, BASSH Spring Meeting, Blackpool https://www.researchgate.net/publication/270567127_Gonorrhoea (slide 30)
- Thin RN, Williams IA, Nicol CS (1971). Direct and delayed methods of immunofluorescent diagnosis of gonorrhoea in women. Br.J.Vener.Dis. 47, 27-30
- Chipperfield EJ, Catterall RD (1976). Reappraisal of Gram-staining and cultural techniques for the diagnosis of gonorrhoea in women. Br.J.Vener.Dis. 52, 36-39
- Evans BA (1976). Detection of gonorrhoea in women. Br.J.Vener.Dis. 52, 40-42
- David Barlow, Nayyar K, Phillips I and Barrow J (1976). Diagnosis of gonorrhoea in women. Br.J.Vener.Dis. 52, 326-328
- David Barlow and Ian Phillips (1978). Gonorrhoea in women: Diagnostic, clinical and laboratory aspects. Lancet; i, 761-4
- Thin RNT and Shaw EJ (1979). Diagnosis of gonorrhoea in women. Br.J.Vener.Dis. 55, 10-13
- Barlow D (2007). (slide 39)
- 11.Turner KME, et al (2014) An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for chlamydia trachomatis and Neisseria gonorrhoeae in genitourinary medicine clinics in England. Sex Transm Infect; 90:104-111
- Barlow D (2007). (Slide 32)
- Belsey EM (1983). Diagnosis of gonorrhoea in women – a national survey. Br.J.Vener.Dis. 59, 59-62
- Mitchell S and Barlow D (1988) unpublished data
- Morton RD (1977) Gonorrhoea. W B Saunders Company Ltd, London, p 41
- Unemo M and Ison C (2013) WHO. Laboratory diagnosis of sexually transmitted infections. Gonorrhoea. p21-54 apps.who.int/iris/bitstream/10665/85343/1/9789241505840_eng.pdf
- Taylor E and Phillips I (1979). Assessment of a selective medium for the isolation of Neisseria gonorrhoeae. Br.J.Vener.Dis. 55, 183-185
- Taylor E and Phillips I (1980). Assessment of transport and isolation methods for gonococci. Br.J.Vener.Dis. 56, 390-393
- Mannion PK, Fairley CK, Fehler G et al (2016). Trends in gonorrhoea positivity by nucleic acid amplification test versus culture among Australian heterosexual men with a low prevalence of gonorrhoea, 2007-2014. Sex Transm Infect 2016; 92:625-628 doi: 10.1136/sextrans-2015-052246