Professor El-Omar has selected Professors Professors Young, Bresalier and Senore (on behalf of the Members of the World Endoscopy Colorectal Cancer Screening New Test Evaluation Expert Working Group) to do the next #GUTBlog.
The #GUTBlog focusses on the paper “An efficient strategy for evaluating new non-invasive screening tests for colorectal cancer: the guiding principles” which was published in paper copy in GUT in October 2023.
Professors Young, Bresalier and Senore (on behalf of the Members of the World Endoscopy Colorectal Cancer Screening New Test Evaluation Expert Working Group) write:
“New non-invasive screening tests for colorectal cancer (CRC) and its precursor lesions are rapidly emerging as novel technologies identify new biomarkers for detecting these neoplasms using a variety of biospecimens. Such tests, many of which are based on panels of biomarkers, may provide opportunities to improve CRC screening programs.
Conducting trials with CRC mortality-reduction as the endpoint to support adoption of a new test can be challenging due to large study size, long duration and cost. Furthermore, what is considered an acceptable level of evidence to justify the use of a new test in population CRC screening varies globally. Thus, the intention has been to provide an efficient, feasible, dynamic and rigorous approach to evaluate emerging tests that is applicable and flexible, whether the screening context is population-based organized screening based on a WHO-style public health model or structured opportunistic screening based on jurisdictional standards of practice.
A formal consensus approach, involving the multidisciplinary expert panel, examined previous evaluation strategies for their suitability in light of emerging new tests, advances in how screening is conducted, and more rigorous analytical specifications and standards. After four rounds of voting, the consensus goal of >80% agreement (agree or strongly agree on a 5-point scale) was achieved for the 12 principles that guide evaluation. Key premises were: screening as a multistep process (from engagement in testing through to treatment), the use of the faecal immunochemical test for haemoglobin (FIT) as the minimum standard for comparison, the need for prospective evaluation in unbiased intended-use screening populations, flexibility to adjust a new test’s positivity threshold to facilitate attainment of a screening program’s goals, and modelling of outcomes in intended-use populations to provide evidence supporting a likely benefit in reducing CRC-mortality and incidence.
The consensus process considered that 12 principles, each with a supporting rationale, were needed to fully consider the challenges presented in evaluating new tests.
The efficacy and effectiveness of a new non-invasive test is efficiently evaluated by comparison to a proven comparator non-invasive test. Quantitative FIT is now considered the appropriate comparator, while colonoscopy remains the diagnostic standard. To be adopted, a new test would need to be shown to be superior to FIT is some way.
A rigorous and efficient four-phased approach to evaluation is proposed (see Figure).
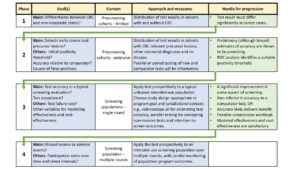
The comprehensive body of information obtained in Phase 3 and 4 findings also provides useful data to inform modelling of test impact on cost-effectiveness, CRC mortality and incidence.
It was considered highly desirable that a new test would be able to meet differing screening goals and regulatory requirements. Such are achieved by providing capacity to adjust a test’s positivity threshold (that triggers follow-up colonoscopy) – even when algorithms are used to interpret biomarker panel information.
It is desirable that a new test has improved accuracy (compared to FIT) for precursor lesions for CRC but more research is needed to identify those precursors that are the most important to detect.
This approach provides comparisons to established comparators and estimates of intermediate outcomes (associated with mortality/incidence) in intended-use populations. The results can be used to model the new test’s impact on these outcomes.
It is expected that these principles will guide researchers, practitioners, regulatory authorities, policy makers and screening program providers in the timely development and validation of a new non-invasive tests.”

Social Media
@FlindersHMRI (for Flinders Health and Medical Research Institute, FHMRI)
@cancerFCIC (for FHMRI Cancer)
@Flinders (Flinders University)
@sdolwani
@tim_kortlever
@tr_levin
@Rockscho
@BobSteele6