Professor El-Omar has chosen Dr Shyam Varadarajulu, Medical Director, Digestive Health Institute, Orlando Health, Orlando, USA to do the next #GUTBlog.
The #GUTBlog focusses on the latest paper “Equivalent performance of single-use and reusable duodenoscopes in a randomised trial” which is due to be published in paper copy in GUT in May 2021. Dr Shyam Varadarajulu is the senior author on this paper.
Dr Varadarajulu writes:
“Given recent outbreaks of duodenoscope-related infection (DRI) transmission involving multidrug resistant organisms, endoscope manufacturers in response to the US FDA mandate have moved to develop single-use duodenoscope platforms. The adoption of this platform is incumbent on two key factors – technical functionality and financial viability.
There are two single-use platforms currently available. The EXALTTM from Boston Scientific (Figure 1) and aSCOPETMDuodeno from Ambu Inc (Figure 2).

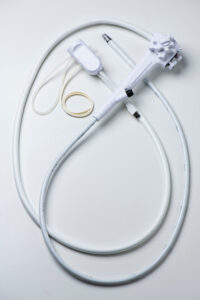
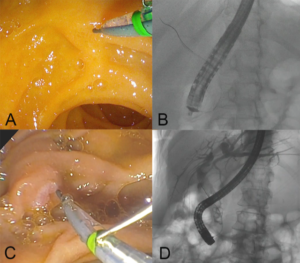
While there is no published clinical data on AMBU, two studies have evaluated EXALTTM. In a series of 73 patients, 96.7% of procedures were completed successfully with no adverse events (1). In a randomized trial, 98 patients underwent ERCP using EXALTTM-D or reusable duodenoscopes (2). While median number of attempts to achieve successful cannulation was less for EXALTTM (2 vs 5, p=0.013) there was no significant difference in rates of cannulation, adverse events, cross-over or need for advanced cannulation techniques. The inherent stiffness of single-use duodenoscope facilitates straight but stable scope position when enface to the duodenal papilla. Consequently, the papilla is engaged from a superior or horizontal angle (figure 3 A,B) rather than from below-upwards (figure 3 C,D) and thereby likely facilitates ductal access in fewer attempts.
Another potential advantage is the shaft stiffness that provides firm anchorage for pulling retrieval balloons in line with bile duct axis thereby making stone extraction easier. Furthermore, passage of accessories such as biopsy forceps and laser fibres during single-operator cholangioscopy is also technically easier as the inherent shaft stiffness straightens the rubber tubing of cholangioscope and facilitates easier passage of accessories through its working channel.
When considering economics related to single-use duodenoscopes, it’s important to look at device cost, reimbursement and associated cost offset by not having to reprocess. The United States Centers for Medicare and Medicaid Services (CMS) recently approved a transitional pass-through payment for outpatient ERCPs performed using single-use duodenoscopes. This is a device specific payment in addition to ERCP procedural payment. Boston Scientific has also submitted an application to CMS for New Technology Add-on Payment for inpatient procedures performed using EXALTTM. If approved, this payment will become available in October 2021. CMS is also reviewing a request for creation of a unique ICD-10-procedure code in reporting use of single-use duodenoscopes for ERCPs performed in inpatient settings.
In addition to improving patient safety by eliminating risk of DRI,the single-use duodenoscope may have a preferential role in high-risk cases such as, immunocompromised patients, known carriers of multidrug-resistant organisms, performance of emergent ERCPs outside of the endoscopy unit and in the event that reusable duodenoscopes are unavailable due to repairs or quarantined awaiting culture results. Form a futuristic perspective, given the nature of manufacturing process, there is greater room for flexibility with single-use duodenoscope design refinements. Therefore, it may be possible to manufacture an ergonomically custom-designed duodenoscope tailored to meet individual needs with additional room for design, performance and technological improvements by providing enhanced mechanics, optics or by incorporating artificial intelligence. The future of ERCP could not be anymore brighter!”
REFERENCES
(1) Muthusamy VR, Bruno MJ, Kozarek RA, Petersen BT, Pleskow DK, Sejpal DV, Slivka A, Peetermans JA, Rousseau MJ, Tirrell GP, Ross AS. Clinical Evaluation of a Single-Use Duodenoscope for Endoscopic Retrograde Cholangiopancreatography. Clin Gastroenterol Hepatol. 2020 Aug;18(9):2108-2117.e3. doi: 10.1016/j.cgh.2019.10.052. Epub 2019 Nov 6. PMID: 31706060.