Professor El-Omar has chosen the prolific team from Leeds, UK, lead by Professor Alexander Ford to do our next, fantastic #GUTBlog.

Professor Ford’s research group has a long-standing interest in the evidence-based management of irritable bowel syndrome (IBS) and published a large number of high impact publications in 2020 already, including in Gut. Dr Christopher Black has worked together with Professor Ford to produce this #GUTblog.

Professor Ford and Dr Black would like to acknowledge Dr Nick Burr who was also instrumental in this work.

“Our research group in Leeds has a long-standing interest in the evidence-based management of irritable bowel syndrome (IBS). Although a single, well-conducted randomised controlled trial (RCT) can provide evidence to support the use of a particular treatment in IBS, we gain a more accurate estimate of treatment efficacy by using meta-analysis, pooling results from similar RCTs together. We have undertaken numerous such trial-based meta-analyses over the years, encompassing a broad range of IBS treatments including dietary interventions, drug treatments, such as antispasmodics and antidepressants, and psychological therapies. These findings have had a wide impact, and have informed guidelines for IBS management.1
Nevertheless, the insights gained from trial-based meta-analyses have limitations. In particular, we cannot infer the relative efficacy of one treatment versus another, information which is important to both patients and doctors when choosing treatments. This problem arises because most trials in IBS only compare an active treatment with a placebo. It is therefore difficult to know whether a new treatment is any better than existing options, or which drug within a certain class should be preferred. Undoubtedly, the best way to resolve this uncertainty would be to conduct high quality head-to-head RCTs of different active treatments. However, it is unlikely that such trials will ever be performed, as they need large numbers of patients to show a benefit of one treatment over another, and would therefore be expensive. Network meta-analysis (NMA) is a technique that can bypass these issues, to some extent.
In simple terms, where two treatments, A and B, share a common comparator, for example a placebo, C, but have not themselves been directly compared in a trial, NMA enables us to estimate the treatment effect between A and B indirectly (Figure 1).
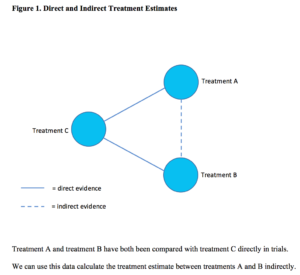
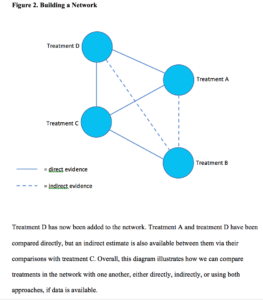
This is because we already know the magnitude and direction of the effect between treatment A and B and the shared comparator, placebo C, from existing trial data. These data are referred to as direct evidence. If we include another treatment, D, (Figure 2) which has been compared with both treatment A, and placebo C, the connections of the network become more complex and, by considering all the direct and indirect treatment estimates together, we can examine the relative efficacy of all included treatments. Furthermore, we can use statistics to rank treatments based on the probability of which one is likely to be the most effective across the network.
The use of NMA to examine the relative efficacy of various treatments in IBS represented the logical progression of our previous work in this field and, over the past 2 years, we have completed several studies. Two of these have been published in Gut, the first of which examined efficacy of licensed treatments for IBS with diarrhoea or mixed stool form.2 Our analysis demonstrated that the 5-hydroxytryptamine-3receptor antagonists alosetron and ramosetron, were likely to be the most effective overall, with alosetron ranked first for effect on stool consistency, ramosetron ranked first for effect on abdominal pain, and alosetron ranked first for a composite endpoint of both parameters. In contrast, although both eluxadoline and rifaximin were more effective than placebo, indirect comparison suggested they were less effective than either alosetron or ramosetron for some endpoints. Unfortunately, alosetron is unavailable outside the USA, and ramosetron is only available in South and South-East Asia. This is unlikely to change, but ondansetron may be a reasonable alternative in the UK and Europe, and an RCT is underway to examine its efficacy.3
Our most recent NMA, investigating the use of psychological therapies in IBS, has also been published in Gut.4 This study examined a range of psychological therapies, including cognitive behavioural therapy (CBT) delivered face-to-face, over the telephone, or via the internet, gut-directed hypnotherapy, individually or in a group setting, and other therapies, including stress management, mindfulness meditation, relaxation therapy, and dynamic psychotherapy. We also assessed the efficacy of the various control interventions used in these trials including routine care, dietary and lifestyle advice, education and support, or a waiting list control, where patients receiving the active intervention are compared with those on a waiting list who will receive it at the end of the trial.
At the first point of follow-up after completing treatment, no psychological therapy was significantly more efficacious than any other active therapy on either direct or indirect comparison. This remained the case when the analysis was restricted to those trials recruiting patients with refractory IBS only. Overall, CBT-based interventions, and gut-directed hypnotherapy, had the largest evidence base. Our findings demonstrated that, of the four control interventions, education and support was likely to be the most effective, suggesting that it should be used as the gold standard in future RCTs of psychological therapies. This would reduce the risk of overestimating the efficacy of psychological interventions in IBS, which might be an issue if, for instance, a waiting list control is used.
We have also published an NMA examining the relative efficacy of licensed drugs for IBS with constipation, including lubiprostone, linaclotide, tenapanor, and plecanatide.5 This demonstrated that all drugs were more effective than placebo, but none was superior to any other on indirect comparison. Linaclotide was the top-ranked treatment, performing well across a range of endpoints, including a composite endpoint of an increase in the number of complete spontaneous bowel movements and improvement in abdominal pain. Finally, we conducted an NMA examining “traditional” therapies for IBS, such as soluble fibre, antispasmodics, peppermint oil, and antidepressants.6 Our findings suggested that peppermint oil may be best for treating global IBS symptoms, whereas tricyclic antidepressants may be best for treating abdominal pain. Both of these NMAs have been published elsewhere.
Overall, NMA is a useful technique for informing an evidence-based approach to managing IBS, and other gastrointestinal conditions, because it allows comparative efficacy to be estimated where few head-to-head trials exist. However, it is not without its own limitations. It cannot overcome bias that might be present in any of the included studies, and there may still be problems with statistical heterogeneity from pooling data. Moreover, we must be careful not to over-interpret treatment rankings; these should serve as a guide only, rather than a definitive pronouncement on the “best” treatment. Nevertheless, until such time as RCTs in IBS follow the example of trials in inflammatory bowel disease and begin to compare newer treatments with existing ones, it is probably the only means of gauging the relative efficacy of therapies for IBS, as we endeavour to offer the highest quality care to our patients with this chronic, and frequently debilitating, condition.”
Dr Christopher Black @DrCJBlack
Dr Nick Burr @Nick_Burr1
Professor Alexander Ford @alex_ford12399
REFERENCES
1. Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am J Gastroenterol 2018;113(Suppl 2):1-18.
3. TRITON: Recruiting patients with diarrhoea predominant IBS for a new trial. 2019 [Available from: https://ctru.leeds.ac.uk/triton (Accessed 18/03/2020)]
5. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of Secretagogues in Patients With Irritable Bowel Syndrome With Constipation: Systematic Review and Network Meta-analysis. Gastroenterology 2018;155(6):1753-63.
6. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2020;5(2):117-31.