By Lisanne Andra Gitsels and Elena Kulinskaya
Cardiovascular disease (CVD) is the biggest cause of death from non-communicable disease in the world.1 Clinical guidelines internationally recommend statin therapy for both primary and secondary prevention of CVD, making statins one of the most frequently prescribed drugs in industrialised countries.2–5 These drugs are usually taken over long time periods to prevent cardiac events, yet little is known about the long-term effects on all-cause mortality. This is because clinical trials are expensive to carry out and therefore tend to be of relatively short duration, with statins trials having on average two to five years of treatment exposure.6–8
Instead, observational studies could contribute to statin research by assessing the effects of prolonged exposure.9 The availability of electronic health records makes it easier to follow up on patients’ health information for an extended period. A 20-year observational follow-up study of the clinical trial WOSCOPS (West of Scotland Coronary Prevention Study) made use of national mortality records and electronic health records to estimate the long-term survival effect of statins.10 The results, however, were unreliable due to major cross-over of the treatment arms with the latest monitoring at five years of follow-up, where more than half of the treatment group had stopped statin therapy while a third of the control group had started the treatment.
In clinical practice, patients are not fixed on a certain treatment regime and can start and stop at any time. Furthermore, clinical guidelines are regularly updated with new evidence from clinical trials and meta-analyses, resulting in new eligibility criteria and treatment courses. This can also be seen in the sequential treatment decisions in managing cardiac risk3 and the alternating statin therapy usage.11 Therefore, there is a need for dynamic survival prediction of long-term time-varying statin therapy.
We previously studied the long-term effects of a history of statin prescription for primary12 and secondary13 prevention of CVD on all-cause mortality using static models at ages 60, 65, 70 and 75 years which did not take account of time-varying covariates after those ages. Whereas a later study showed that current but not former use of statins is effective for the prevention of CVD.14 Our latest work, therefore, assessed whether current statin prescription for primary and secondary prevention of CVD reduces mortality in the general population, with updated survival predictions every six months from age 60 to 85 years reflecting fluid clinical practice.
In medicine, the Cox proportional hazard regression is the go-to method for time-to-event data, i.e. survival analysis, because of not having to specify the baseline hazard, its transparency in regards to what is being compared and therefore its simplicity in interpreting the findings. There are multiple improved versions of this regression and the two main approaches that allow for time-dependent covariates are time-dependent Cox regression and landmark analysis.15 In time-dependent Cox regression, it is assumed that “the value for the time-dependent covariate is known for all subjects at all event time points” such that valid inferences can be made,15 in our case that the presence or absence of current statin prescription is known for the entire sample at any time death is experienced in this population. This information might be available in electronic health records, however it is extremely labour intensive to pull out the information when having a large sample size, in our case over 110 000 patients.
In landmarking, the standard Cox regression model is fitted at a time called “landmark” using the latest information of the covariate at that time to predict survival for a set length beyond that time called “window”.16 This will be done multiple times at multiple landmarks, creating a sliding window of updated survival predictions.16 For example, our study included people who turned 60 years old and had no statin prescription, our first landmark point was at age 60.5 and the prediction window at 5 years. Then the presence or absence of current statin prescription at age 60.5 was used to predict survival in the next 5 years until age 65.5 (see Figure 1). The next landmark was six months later at age 61 years, and so statin prescription at age 61 was used to predict the 5-year survival until age 66. The advantages of landmarking are that it allows for time-dependent covariates (i.e. current statins prescription) as well as time-dependent effects (i.e. the survival benefit of being prescribed statins). Furthermore, landmarking can predict beyond the study period given enough data, and is transparent in what is being compared at each landmark. With landmark analysis, survival can be visualised by plotting the Kaplan-Meier survival curves for the risk groups of interest to provide information about the absolute and relative risks, whereas this is not straightforward for time-dependent Cox regression.16
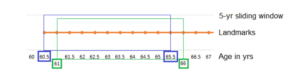
Figure 1. Landmarking process of 5-year survival predictions (“sliding window”) updated every six months (“landmarks”).
Although some landmark analyses can be accused of randomly chosen time points,16 our study chose landmarks every six months because NHS guidelines recommend reviewing repeat prescriptions every six to 12 months.17 Another disadvantage of landmarking is the potential loss of statistical power because events before the landmark time point or after the prediction window are excluded from the analysis.16 However, that was not a concern in our study utilising 1.8 million person-years of routinely collected primary care data.
We fitted Cox regression models to predict 5-, 10-, and 25-year survival associated with current statin prescription at each landmark (every six months) from age 60 to 85 years, creating a sliding time window at 51 landmarks. We then translated the 10-year survival predictions to absolute and relative risk reduction at key ages by various risk profiles to inform decision making in clinical guidelines. The profiles differentiated by sex, year of birth, cardiac risk, health status and deprivation. Our study found that current statin prescription was associated with significant absolute and relative reductions in mortality from age 65 onward, irrespective of sex and cardiac risk. The novelty of our findings is that statins are not only beneficial at a static moment but can be initiated and continued at older ages with long-term survival benefits. This supports the use of statins at older ages where clinically indicated and after discussion of the potential risks and benefits with the patient.
References
- Gakidou E, Afshin A, Abajobir AA, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345-1422.
- Moody A, Mindell J, Faulding S. Health Survey for England 2016: Prescribed Medicines.; 2017. www.statisticsauthority.gov.uk/assessment/code-of-practice
- National Institute for Health and Care Excellence. Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. 2014.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
- Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590.
- Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ. 2009;339(7711):36.
- Armitage J, Baigent C, Barnes E, et al. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407-415.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532-2561.
- Vallejo-Vaz AJ, Robertson M, Catapano AL, et al. Low-Density Lipoprotein Cholesterol Lowering for the Primary Prevention of Cardiovascular Disease Among Men With Primary Elevations of Low-Density Lipoprotein Cholesterol Levels of 190 mg/dL or Above. Circulation. 2017;136(20):1878-1891.
- Ofori-Asenso R, Jakhu A, Zomer E, et al. Adherence and Persistence Among Statin Users Aged 65 Years and Over: A Systematic Review and Meta-analysis. Journals Gerontol Ser A. 2018;73(6):813-819.
- Gitsels LA, Kulinskaya E, Steel N. Survival Benefits of Statins for Primary Prevention: A Cohort Study. PLoS One. 2016;11(11):e0166847.
- Gitsels LA, Kulinskaya E, Steel N. Survival prospects after acute myocardial infarction in the UK: A matched cohort study 1987-2011. BMJ Open. 2017;7(1).
- Jun JE, Cho IJ, Han K, et al. Statins for primary prevention in adults aged 75 years and older: A nationwide population-based case-control study. Atherosclerosis. 2019;283:28-34.
- Putter H, van Houwelingen HC. Understanding Landmarking and Its Relation with Time-Dependent Cox Regression. Stat Biosci. 2017;9(2):489-503.
- van Houwelingen HC. Dynamic Prediction by Landmarking in Event History Analysis. Scand J Stat. 2007;34(1):70-85.
- Mahtani KR, MacKenna B, Spencer E. Should we prescribe longer repeat prescriptions for patients with long-term conditions during a pandemic? The Centre for Evidence-Based Medicine (CEBM). Published 2020. https://www.cebm.net/covid-19/should-we-prescribe-longer-repeat-prescriptions-for-patients-with-long-term-conditions-during-a-pandemic/
Conflict of Interest: None declared